ʵ���ҳ���Ũ�������������Ϊ36.5%���ܶ�Ϊ1.20g/cm
-3������100mL 3.00mol/L��ϡ���ᣬ������Ũ����
25ml
25ml
��
��1����������Һʱʵ����Ӧ�ṩ�ձ�����Ͳ��100ml����ƿ��
����
����
��
��ͷ�ι�
��ͷ�ι�
��
��2��ʹ������ƿǰ������е�һ��������
��©
��©
��3����Ũ�������Ƹ�ϡ������Ҫ������Щ���裨������˳����д��ţ���
�٢ۢޢݢܢߢ��
�٢ۢޢݢܢߢ��
��
�ټ��� �ڣ�װƿ �ۣ���50mL��Ͳ��ȡһ�������Ũ����
��ϴ�� �ݣ���Һ �ޣ�ϡ�� �ߣ����� �࣮ҡ�� �ᣮ��
��4�������ƹ�����������������ȷ�����в���������������ҺŨ��ƫС����
�٢�
�٢�
��û��ϴ���ձ��Ͳ�������������ƿ�к�����������ˮ���۶���ʱ���ӱ��ߣ��ܶ���ʱ���ӱ��ߣ�
��5��MnO
2 ��Ũ����ķ�Ӧ�У� MnO
2+4HCl��Ũ��
MnCl
2+Cl
2��+2H
2O ���������34.8g MnO
2 ����ԭ����������HCl��
29.2
29.2
�ˣ����������ڱ���µ����
8.96
8.96
L��




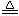 MnCl2+Cl2��+2H2O
MnCl2+Cl2��+2H2O