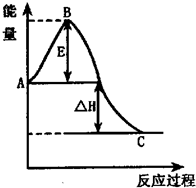
�����Ͱ�����������̼�����Դ��
��1��������Գ���ر���Ϊ����ɫ��ѧ��Դ�����š����ʱ�ķ�Ӧ��2NiOOH+H
22Ni��OH��
2���ŵ�ʱ�������ĵ缫��ӦʽΪ
NiOOH+e-+H2O=Ni��OH��2+OH-
NiOOH+e-+H2O=Ni��OH��2+OH-
�����ʱ���õ缫Ӧ���Դ��
��
��
���������������������
��2�����ڿ�����ȼ�գ�����ˮ�͵�������֪��
N
2��g��+3H
2��g��?2NH
3��g������H=-92.4kJ?mol
-12H
2��g��+O
2��g��=2H
2O��l������H=-572kJ?mol
-1���ڿ�����ȼ������Һ̬ˮ�͵���ʱ���Ȼ�ѧ����Ϊ��
4NH3��g��+3O2��g��=2N2��g��+6H2O��l������H=-1531.2kJ?mol-1
4NH3��g��+3O2��g��=2N2��g��+6H2O��l������H=-1531.2kJ?mol-1
��
��3���о�������ҵ�Ϻϳɰ���Ӧ ��N
2+3H
22NH
3�� ��25�桢400���ƽ�ⳣ���ֱ�Ϊ5��10
5��200��
�ٺϳɰ���
����
����
��Ӧ������ȡ������ȡ�����
�ںϳɰ�ѡ��400��500���ԭ���ǣ�
�ӿ췴Ӧ���ʣ������������
�ӿ췴Ӧ���ʣ������������
��
�����ݻ��̶����ܱ������з���������Ӧ���±���Ϊ�������ڲ�ͬʱ�̵�Ũ�ȣ�
| ʱ��/min |
c��N2��/mol?L-1 |
c��H2��/mol?L-1 |
c��NH3��/mol?L-1 |
| 0 |
0.6 |
1.8 |
0 |
| 5 |
0.48 |
X |
0.24 |
| 10 |
0.26 |
0.78 |
0.68 |
0��5min��H
2��ƽ����Ӧ����v��H
2��=
0.072mol/L��min
0.072mol/L��min
����Ӧ��5����ʱ�����������˸ı䣬�ı������������
a
a
������ţ���
a��ʹ�ô��� b�������¶� c������������Ũ�� d�������NH
3��4����-50��ʱ��Һ���д��ڵ���ƽ��NH
3��l��?NH
4++NH
2-�����ӻ�����
K=c��NH
4+��?c��NH
2-������һ�������£�ƽ��ʱc��NH
2-��=1��10
-15mol?L
-1��
����˵����ȷ���ǣ�
a����Һ���м���NaNH
2��Һ�������ӻ���������
b�����¶���Һ�������ӻ�����Ϊ1��10
-30c��Һ����-50��ĵ���̶ȱȳ����´�ˮ�Ĵ�



 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�




 ��2010?�ϲ�һģ���ں��º��ݵ��ܱ�������ͨ��1molN2��XmolH2���������·�Ӧ��N2��g��+3H2��g��
��2010?�ϲ�һģ���ں��º��ݵ��ܱ�������ͨ��1molN2��XmolH2���������·�Ӧ��N2��g��+3H2��g��