
п�������ǻ��ý����������������������ǿ�ᣬ��������ǿ������������������ڰ�ˮ����������п�����ڰ�ˮ������[Zn(NH3)4]2����
�ش��������⣺
(1)��������������������Һ����Һ����Ԫ�صĴ�����ʽΪ________________(�û�ѧʽ��ʾ)��
(2)д��п������������Һ��Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________________________________________________________________________________��
(3)���и����е�������Һ������μӵ�ʵ�鷽�����ɼ������________________��
�����������������ơ����������Ͱ�ˮ��������п���������ơ�������п�Ͱ�ˮ
(4)д�������������백ˮ��Ӧ�����ӷ���ʽ��________________________________________________________________________________________________________________________________________________��
�Խ�����ʵ���Ҳ������ÿ�����п���백ˮ��Ӧ�Ʊ�������п��ԭ��_____________________________________________________________________________________________________________________________��
������(1)Al��NaOH��Һ������Ӧ�Ļ�ѧ����ʽΪ��2Al��2NaOH��6H2O===2NaAlO2��3H2������Һ��Al��AlO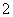 ��ʽ���ڡ�
��ʽ���ڡ�
(2)����Al��NaOH��Һ�ķ�Ӧ������д��Zn��NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ��Zn��2NaOH===Na2ZnO2��H2����
(3)������Al2(SO4)3��Һ����NaOH��Һ��û�г���������AlO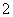 ������NaOH��Һ����Al2(SO4)3��Һ���г�����
������NaOH��Һ����Al2(SO4)3��Һ���г�����
��Al2(SO4)3��Һ���백ˮ���г�������ˮ����Al2(SO4)3��Һ��Ҳ�г�����
������ZnSO4��Һ����NaOH��Һ��û�г���������ZnO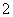 ������NaOH��Һ����ZnSO4��Һ���г�����
������NaOH��Һ����ZnSO4��Һ���г�����
������ZnSO4��Һ���백ˮ��û�г���������[Zn(NH3)4]2����������ˮ����ZnSO4��Һ���г�����
���ԣ�����μӵ�ʵ�鷽�����ܼ�����Т٢ۢܡ�
(4)���ݸ�����Ϣ��д����ѧ����ʽ��
Al3����3NH3·H2O===Al(OH)3����3NH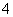
������п���백ˮ��Ӧ������Zn(OH)2�����ڹ�����ˮ������[Zn(NH3)4]2��������ʵ������м��백ˮ�����������ơ�
�𰸡�(1)AlO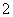
(2)Zn��2NaOH===Na2ZnO2��H2��
(3)�٢ۢ�
(4)Al3����3NH3·H2O===Al(OH)3����3NH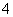 ��������п���백ˮ��Ӧ������Zn(OH)2�����ڹ�����ˮ�У�����[Zn(NH3)4]2������ˮ������������
��������п���백ˮ��Ӧ������Zn(OH)2�����ڹ�����ˮ�У�����[Zn(NH3)4]2������ˮ������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и��������У���ѧ��������ȫ��ͬ����(����)
A��HI��NaI B��H2S��CO2
C��Cl2��CCl4 D��F2��NaBr
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������[(NH4)xNiy(SO4)m·nH2O]�����ڵ�ơ�ӡˢ������ijͬѧΪ�ⶨ������淋���ɣ���������ʵ�飺��ȷ��ȡ2.335 0 g��Ʒ�����Ƴ�100.00 mL��ҺA����ȷ��ȡ25.00 mL��ҺA����0.040 00 mol·L��1��EDTA(Na2H2Y)����Һ�ζ����е�Ni2��(���ӷ���ʽΪNi2����H2Y2��===NiY2����2H��)������EDTA����Һ31.25 mL������ȡ25.00 mL��ҺA����������NaOH��Һ����ּ��ȣ�����NH3 56.00 mL(��״��)��
(1)���ζ�����ʹ��ǰδ��EDTA����Һ��ϴ����õ�Ni2��������________(�ƫ�ߡ�����ƫ�͡����䡱)��
(2)��������________���飬������______________________________________________��
(3)ͨ������ȷ��������淋Ļ�ѧʽ(д���������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���в��ֱ�����������Ʒ10 g������Ͷ��ˮ�г�ַ�Ӧ����������Һϡ�ͳ�400 mL����ʵ���ø�����Ʒ�к���8%����Ԫ�أ�������������Һ��pHΪ(����)
A��14 B��13 C��12 D��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и�������У����������Ȳ���������Ȼ��������ܽ⡱������Ǣ���̼������Һ��ͨ�������CO2������NaAlO2��Һ����μ��������ϡ�������AlCl3��Һ����μ��������ϡ����������Һ�������������Һ����μ������������
A���٢� B���٢�
C���٢� D���ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪�����Ȼ�ѧ����ʽ��
��H2(g)��1/2O2(g)===H2O(g)����H1��a kJ·mol��1
��2H2(g)��O2(g)===2H2O(g)����H2��b kJ·mol��1
��H2(g)��1/2O2(g)===H2O(l)����H3��c kJ·mol��1
��2H2(g)��O2(g)===2H2O(l)����H4��d kJ·mol��1
���й�ϵʽ����ȷ����(����)
A��a<c<0���������������� B��b>d>0
C��2a��b<0 D��2c��d>0
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ������50 mL 0.50 mol·L��1���ᡢ50 mL 0.55 mol·L��1 NaOH��Һ����ͼ��ʾװ�ý��вⶨ�к��ȵ�ʵ�飬�õ����е����ݣ�
| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | |
| ���� | NaOH��Һ | ||
| 1 | 20.2 | 20.3 | 23.7 |
| 2 | 20.3 | 20.5 | 23.8 |
| 3 | 21.5 | 21.6 | 24.9 |
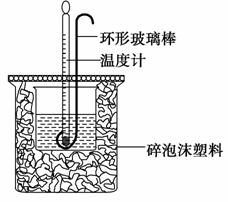
������������⣺
(1)ʵ��ʱ�û��β�����������Һ�ķ�����________________________________________________________________
_______________________________________________________________��
������ͭ˿��������滷�β�������������_______________________________________________________________��
(2)�����ݴ�����t2—t1��3.4 �档���ʵ���õ��к��Ȧ�H��________[�����NaOH��Һ���ܶȰ�1 g·cm��3���㣬��Ӧ������Һ�ı�����(c)��4.18 J·(g·��)��1����]��
(3)����NaOH��Һ��Ϊ��ͬ�������ͬŨ�ȵİ�ˮ������к���Ϊ��H1����H1�릤H�Ĺ�ϵΪ����H1________��H(�����������������)��������________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һС������Ʒֱ�Ͷ��ʢ�У�a.ˮ��b.�Ҵ���c.ϡH2SO4������С�ձ��У���Ӧ�����ɿ쵽����˳��Ϊ______________�����ͷ�Ӧ���ʲ�ͬ��ԭ��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ��ʹ�Ա�ڷɴ��еõ�һ���ȶ��ġ����õ����滷����һ���ڷɴ��ڰ�װʢ��Na2O2��K2O2������װ�ã�������;�Dz������������й���Na2O2��������ȷ����(����)
A��Na2O2�����������ӵĸ�����Ϊ1��1
B��Na2O2�ֱ���ˮ��CO2��Ӧ������ͬ����O2ʱ����Ҫˮ��CO2���������
C��Na2O2�ֱ���ˮ��CO2��Ӧ������ͬ����O2ʱ��ת�Ƶ��ӵ����ʵ������
D��Na2O2��Ư��ԭ����SO2��Ư��ԭ����ͬ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com