


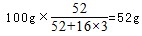 ����B��ʱ���������Ϊ��100g��76%=76g��Co������û�б䣬������������Co������Ϊ52g����Ԫ�ص�����Ϊ16�����ߵĸ�����Ϊ
����B��ʱ���������Ϊ��100g��76%=76g��Co������û�б䣬������������Co������Ϊ52g����Ԫ�ص�����Ϊ16�����ߵĸ�����Ϊ ������B��ʱʣ�����ijɷ���Cr2O3���ʴ�Ϊ��Cr2O3��
������B��ʱʣ�����ijɷ���Cr2O3���ʴ�Ϊ��Cr2O3��

 �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д� Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������[NH4Al(SO4)2��12H2O]��Һ�м������Ba(OH)2��Һ�� Al3+��2SO42����2Ba2+��4OH����AlO2����2BaSO4����2H2O |
| B��H218O��Ͷ��Na2O2���壺2H218O + 2Na2O2 = 4Na+ + 4OH- + 18O2�� |
| C��̼�������Һ�мӹ�������ʯ��ˮ��Ca2+ + OH- + HCO3- = CaCO3�� + H2O |
D��̼���Ƶ�ˮ�ⷴӦ��CO32��+ H3O�� HCO3��+ H2O HCO3��+ H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A�����ҽ���Һ�м������ᡢ˫��ˮ��2I-��2H+��H2O2 I2��2H2O I2��2H2O |
B��CuƬ����FeCl3��Һ�У�Cu��Fe3+ Fe2++Cu2+ Fe2++Cu2+ |
C����Al2(SO4)3��Һ�м�������İ�ˮ�� Al3����4NH3��H2O  AlO2-��4NH4+��2H2O AlO2-��4NH4+��2H2O |
| D���� NaHSO4��Һ�еμ�Ba(OH)2��Һ�����ԣ� |
 BaSO4����H2O
BaSO4����H2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������Ͷ�뵽NaOH��Һ�У�2Al+2OH��=2AlO2��+H2�� |
| B��AlCl3��Һ�м��������İ�ˮ��Al3++ 3OH�� =Al(OH)3�� |
C�����Ȼ�����Һ�м������ۣ� |
| D��FeCl2��Һ��Cl2��Ӧ��2Fe2++Cl2=2Fe3++2Cl�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NH4HCO3���ڹ�����ŨKOH��Һ�У�NH4++ OH-= NH3��+ H2O |
| B����������Һ�еμ�Ba(OH)2��Һ��ǡ��ʹSO42-������ȫ��2Al3++3SO42-+3Ba2++6OH -="2" Al(OH)3��+3BaSO4�� |
| C����FeBr2��Һ��ͨ������������2Fe2++4Br-+3Cl2="2" Fe3++2 Br2+6 Cl- |
| D�������ȥˮ����2H++CaCO3=Ca2++ CO2��+ H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��KCl+AgNO3��AlCl3+ AgNO3 |
| B��NaHCO3+H2SO4��Na2CO3+HCl |
| C��NaHCO3+NaOH��Ca��HCO3��+KOH |
| D��BaCl2+H2SO4��Ba(OH)2+H2SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��̼��������Һ�е�������������Һ��HCO3�D + OH�D �� CO32�D�� H2O |
| B����������ͨ�����������Һ��SO2 + ClO�D + 2OH�D�� SO42�D��Cl�D��H2O |
| C��������ϡ���BaS + 2H���� H2S��+ Ba2+ |
| D����������Һ�������Һ��ϣ�SiO32��+2H����H2SiO3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������ϡ���ᣬ��Һ���: 3 Fe +8H+ +2NO3-="3" Fe2++2NO+4H2O |
| B��FeBr2��Cl2���ʵ���֮��Ϊ1��1ʱ:2Fe2++2Br-+2Cl2=2Fe3++4C1-+Br2 |
| C����������Һ�еμ�����������Һ��SO42-��ȫ����:A13++2SO42-+2Ba2++4OH-=2BaSO4��+A1O2-+2H2O |
| D��Ư����Һ�м��Ȼ�����Һ�����������ɫ����:Fe3++3C1O-+3H2O=Fe(OH)3��+3HC1O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��������������������Fe3O4��8H�� 2Fe3����Fe2����4H2O 2Fe3����Fe2����4H2O |
B���ù�����ˮ���չ�ҵβ���е�SO2��2NH3��H2O��SO2 2NH4����SO32����H2O 2NH4����SO32����H2O |
C����Ũ�����ữ�ĸ��������Һ��������ⷴӦ��2MnO4�� +5H2O2 + 6H+ 2Mn2+ +5O2�� + 8H2O 2Mn2+ +5O2�� + 8H2O |
D���ð�ˮϴ���Թ��ڱڵ�������Ag����2NH3��H2O [Ag��NH3��2]++2H2O [Ag��NH3��2]++2H2O |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com