

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��1 mol��L��1 NaCl��Һ����NA��Na�� |
B��1 mol Cu������ϡ���ᷴӦ����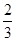 NA��NO���� NA��NO���� |
| C��1 L 0.1 mol��L��1��ˮ����0.1NA��OH�� |
| D��1 mol Fe2����������H2O2��Һ��Ӧ��ת��2NA������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��78g�������ƾ����У���2 NA�������� |
| B��a gO2��O3�Ļ�������к��еķ�������ΪaNA��32 |
| C�����³�ѹ�£�28 gCO��N2�Ļ�������к��еķ�������ΪNA |
| D����״���£�22��4 L H2S��SO2�Ļ�������к��еķ�������ΪNA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����״���£�22.4 L�Ҵ��к��е�̼ԭ����ĿΪ2NA |
| B��1 mol CnH2n���еĹ��õ��Ӷ���Ϊ(3n��1)NA |
| C��1 mol CO2��1 mol Na2O2��ȫ��Ӧʱ��ת�Ƶĵ�����ĿΪNA |
| D��1 mol��L��1��FeCl3��Һ�У�����Fe3������ĿС��NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ڱ���£�11.2 L NO��11.2 L O2��Ϻ�����������Ϊ0.75NA |
| B�����³�ѹ�£�16 g O3�����ĵ�����Ϊ8NA |
| C��0.1 mol Na2O2�������0.4NA������ |
| D����������������Һ��Ӧ����1 mol����ʱ��ת�Ƶĵ�����ΪNA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����100 mL 3 mol��L��1��H2SO4��100 mL H2O��ϣ���������ʵ���Ũ�ȸı�Ϊ1.5 mol��L��1 |
| B����200 mL 3 mol��L��1��BaCl2��Һ��100 mL 3 mol��L��1��KCl��Һ��Ϻ���Һ�е�c(Cl��)��Ȼ��3 mol��L��1 |
| C����100 g 20%��NaCl��Һ��100 g H2O��Ϻ�NaCl��Һ������������10% |
| D����100 mL 20%��NaOH��Һ��100 mL H2O��Ϻ�NaOH��Һ������������10% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��һ������Fe�뺬1 mol HNO3��ϡ����ǡ�÷�Ӧ����ԭ�ĵ�ԭ����С��NA |
| B��1 mol Na2O2�����к���������Ϊ4NA |
| C�����³�ѹ�£���������SO2��S2������ͬ�ķ����� |
| D��125 g CuSO4��5H2O�������0.5NA��Cu2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��7.8��Na2S��Na2O2�Ĺ��������к��е���������ĿΪ0.1NA |
| B����ͭ���缫���CuSO4��Һ�ķ�Ӧ����ʽ�� 2Cu2����2H2O  2Cu��O2����4H�� 2Cu��O2����4H�� |
| C������ʱ��Ũ�Ⱦ�Ϊ0.01mol/L�� Na2CO3��Һ��NaHCO3��Һ�������ϣ�����Һ������Ũ�ȹ�ϵΪ c(CO32��) ��c(HCO3��)��c(H2CO3) ��0.02mol/L |
| D��������ˮ�еμ�ŨH2SO4��KW���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com