���
�⣺��1��CO��ȼ����Ϊ283kJ/mol����CO����������Ӧ���Ȼ�ѧ����ʽΪCO��g��+
O
2��g���TCO
2��g����H=-283kJ/mol��
H
2��ȼ����Ϊ285.8kJ/mol����H
2��������Ӧ���Ȼ�ѧ����ʽΪH
2��g��+
O
2��g���TH
2O��l����H=-285.8kJ/mol��
��ͼ1��֪��CO��H
2��Ӧ���Ƶü״����Ȼ�ѧ����ʽΪ��CO��g��+2H
2��g���TCH
3OH��l����H=-129kJ/mol��
���ݸ�˹���ɣ���CH
3OHȼ���ȵ��Ȼ�ѧ����ʽ�ɢ�+�ڡ�2-�ۿɵã�
CH
3OH��l��+
O
2��g��=CO
2��g��+2H
2O��l����H=-725.6mol/L��
�ʴ�Ϊ��CH
3OH��l��+
O
2��g��=CO
2��g��+2H
2O��l����H=-725.6mol/L��
��2������ƽ��ʱCO��Ũ��Ϊ0.25mol/L��֪����Ӧ���ĵ�H
2���ʵ���Ϊ2��2L��0.75mol/L=3mol����ƽ��ʱH
2�����ʵ���Ũ��Ϊ0.5mol/L������ƽ�ⳣ���������ɵ�Ũ����֮�����Է�Ӧ���Ũ����֮���ɵã�
k=
=
| 0.75mol/L |
| 0.25mol/L��(0.5mol/L)2 |
=12��mol/L��
-2��
ƽ��ʱn��CO��=0.5mol��n��H
2��=1mol��n��CH
3OH��=1.5mol����3mol��
��ʼʱ��n��CO��=2mol��n��H
2����=4mol����6mol��
���ݰ����ӵ����ɿ�֪ѹǿ֮�ȵ������ʵ���֮�ȣ�10min�������ڵ�ѹǿ��Ϊԭ����
����
A��������Ӧ���ȣ������¶ȿ�ʹƽ��������Ӧ�����ƶ������CO��ת���ʣ���A��ȷ��
B���Ӵ�����ƽ�ⲻ�ƶ���ת���ʲ��䣬��B����
C�����������ʹ��ϵѹǿ�����ڲμӷ�Ӧ��������˵��Ũ�Ȳ��䣬ƽ�ⲻ�ƶ���ת���ʲ��䣬��C����
D���ٳ���1molCO��2molH
2ѹǿ����ƽ��������Ӧ�����ƶ������CO��ת���ʣ���D��ȷ��
E�����º��ݸ�Ϊ���º�ѹ��ѹǿ�Ϻ���ʱ������ѹǿ��ƽ��������Ӧ�����ƶ������CO��ת���ʣ���E��ȷ��
�ʴ�Ϊ��12��mol/L��
-2��0.5��ADE��
��3����0.1mol/L��HCOOH��Һ�еμ�NaOH��Һ�����۷�Ӧ�����Σ���Һ�о��е���غ㣺C��OH
-��+c��HCOO
-��=c��Na
+��+C��H
+��������Һ��c��HCOO
-����c��Na
+�����ʿ�֪��Һ��C��OH
-����C��H
+������Һ�ʼ��ԣ�
A��NaOH���㣬HCOOH�м�����ʣ��ʱ����Һ���Գʼ��ԣ���Aѡ��
B��HCOOH��NaOHǡ����ȫ��Ӧ����ȫ����HCOONaʱ������HCOONa��ǿ�������Σ�ˮ���Լ��ԣ���Bѡ��
C��NaOH����ʱ����Һһ���Լ��ԣ���Cѡ��
��ѡABC��

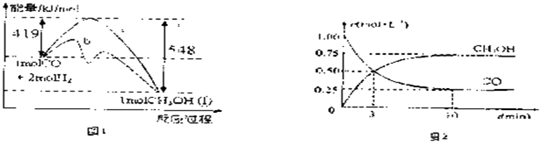



 ������Ԫ��A��B��C��D�����ڱ���λ����ͼ��ʾ������Ԫ��Dԭ���������3�����ӣ�
������Ԫ��A��B��C��D�����ڱ���λ����ͼ��ʾ������Ԫ��Dԭ���������3�����ӣ�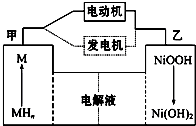 ��������϶������������õ綯������ȼ������߽���ƶ����֣��������»����ʱ���綯���ṩ�ƶ������������͵����ģ�
��������϶������������õ綯������ȼ������߽���ƶ����֣��������»����ʱ���綯���ṩ�ƶ������������͵����ģ�