
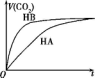
 �������Ũ�Ⱦ�Ϊ0.2mol•L-1��������Һ����HA��Һ����HB��Һ����NaHCO3��Һ����֪���٢ڷֱ���ۻ�ϣ�ʵ������������CO2���������V����ʱ�䣨t���仯��ͼ��ʾ������˵������ȷ���ǣ�������
�������Ũ�Ⱦ�Ϊ0.2mol•L-1��������Һ����HA��Һ����HB��Һ����NaHCO3��Һ����֪���٢ڷֱ���ۻ�ϣ�ʵ������������CO2���������V����ʱ�䣨t���仯��ͼ��ʾ������˵������ȷ���ǣ�������| A�� | ���ԣ�HB��HA��H2CO3 | |
| B�� | ��Ӧ��������������Һ�У�c��A-����c��B-�� | |
| C�� | ����������Һ����ˮ�����c��OH-����Դ�С��NaHCO3��HA��HB | |
| D�� | HA��Һ��NaHCO3��Һ��Ӧ���õ���Һ�У�c��A-��+c��HA��=0.1 mol•L-1 |
���� A��HB��NaHCO3��Һ��Ӧ���ʿ죬��Һ��������Ũ�ȴ�
B���������ˮ��̶�Խ��Ũ��ԽС��
C���������Խǿ����ˮ�ĵ������Ƴ̶�Խ��
D���������Ϻ��������һ����Ũ��Ϊԭ����һ�룮
��� �⣺A����ͼ��֪��HB��NaHCO3��Һ��Ӧ���ʿ죬��Һ��������Ũ�ȴ�����HB�����Դ���HA�������ԵıȽϣ�HB��HA��H2CO3����A��ȷ��
B�����ԣ�HB��HA����HA���������ˮ��̶ȴ�Ũ��С����c��A-����c��B-������B����
C��NaHCO3ˮ��ٽ�ˮ�ĵ��룬�������Խǿ����ˮ�ĵ������Ƴ̶�Խ������HB��ˮ�����Ƴ̶������������Һ����ˮ�����c��OH-����С��NaHCO3��Һ��HA��Һ��HB��Һ����C��ȷ��
D��HA��Һ��NaHCO3��Һ��Ӧ������Һ���������һ����Ũ��Ϊԭ����һ�룬����c��A-��+c��HA��=0.1mol/L����D��ȷ��
��ѡB��
���� ���⿼����������ʵĵ���ƽ��Ӧ�ã�ͼ�����������ˮ�ĵ���Ӱ�����أ���Ҫ��������ǿ���ıȽϣ�����ˮ���Ӧ�ã��������Һ��Ԫ���غ��Ӧ�ã���Ŀ�Ѷ��еȣ�


 �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

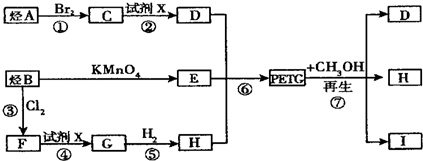
 $\stackrel{KMnO_{4}}{��}$
$\stackrel{KMnO_{4}}{��}$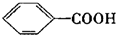
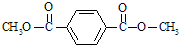 ��
��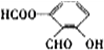 ��
�� ��
��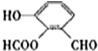 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ƾ� | B�� | ���ᣨ���ᣩ | C�� | ���Ȼ�̼ | D�� | ŨH2SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 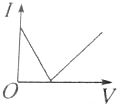 | B�� | 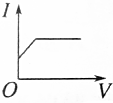 | C�� |  | D�� |  |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
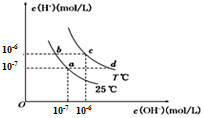 �ڲ�ͬ�¶��£�ˮ��Һ��c��H+����c��OH- ������ͼ��ʾ��ϵ������˵����ȷ���ǣ�������
�ڲ�ͬ�¶��£�ˮ��Һ��c��H+����c��OH- ������ͼ��ʾ��ϵ������˵����ȷ���ǣ�������| A�� | c���Ӧ����ҺpH=6����Һ�������� | |
| B�� | b���Ӧ����Һ�п��Դ������棺NH4+��K+��CO32-��Cl- | |
| C�� | ��a���ʾCH3COOH��CH3COONa�Ļ����Һ�������Һ�е�����Ũ�ȴ�СΪ��c��Na+ ����c��CH3COO-����C��OH- ����c��H + �� | |
| D�� | ��d���ʾij��Ũ�ȡ��������NH3•H2O��NH4Cl�����Һ������Һ�е�����Ũ�ȴ������¹�ϵ��c��NH4+��+2c��H+���T2c��OH-��+c��NH3•H2O�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Һ��Pb2+��I-Ũ�ȶ����� | B�� | �ܶȻ�����Ksp���� | ||
| C�� | �����ܽ�ƽ���������ƶ� | D�� | PbI2���ܽ�Ȳ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | SO2+H2O�TH2SO3 | B�� | H2CO3�TCO2��+H2O | ||
| C�� | 2NaOH+H2SO4�TNa2SO4+2H2O | D�� | C+O2$\frac{\underline{\;��ȼ\;}}{\;}$CO2 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com