
���������ַ��������Ƶð�ɫ��Fe��OH��2������
����һ���ò���Fe3+��FeSO4��Һ�벻��O2������ˮ���Ƶ�NaOH��Һ��
��1������������������������FeSO4��Һʱ�������_____________��
��2����ȥ����ˮ���ܽ��O2������_____________�ķ�����
��3�����ɰ�ɫFe��OH��2������Ҫ�ó��ι���ȡ����O2��NaOH��Һ������FeSO4��ҺҺ���£��ټ���NaOH��Һ������������������_________________________________________��
��������������ͼװ���У���NaOH��Һ����м��ϡH2SO4���Լ��Ʊ���
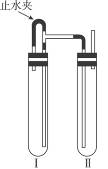
��1�����Թܢ��������Լ���________________��
��2�����Թܢ��������Լ���________________��
��3��Ϊ���Ƶð�ɫFe��OH��2���������Թܢ�͢��м����Լ�����ֹˮ�У��������Ӻ��ʵ�鲽����_________________________________��
��4���������ɵ�Fe��OH��2�����ܽϳ�ʱ�䱣�ְ�ɫ����������______________________��
����һ����1��ϡH2SO4����м
��2�����
��3���������ɵ�Fe��OH��2�����Ӵ�O2
����������1��ϡH2SO4����м
��2��NaOH��Һ
��3�������Թܢ���ڴ��ų��������Ĵ��ȣ����ų���H2����ʱ���ټн�ֹˮ��
��4���Թܢ��з�Ӧ���ɵ�H2�������Թܢ���Թܢ�����������������
����һ����1��Fe2+��ˮ�⣬Ҫ����ϡH2SO4������ˮ��̶ȣ�Fe2+�ױ�������O2������Ҫ��Fe�ۣ�����������Fe3+��ԭ��Fe2+��
��2��������ܽ�����¶����߶����ͣ�������к��ˮ���ܽ��O2���١�
����������3����֧�Թܣ��Թܢ��ǿ���ʽ�����ò��������彫��Һѹ���Թܢ��У�������ȡFeSO4Ӧ�����Թܢ��У������Թܢ���ʢ����O2��NaOH��Һ����Fe��ϡH2SO4�����Թܢ������H2�ɴ��Թܢ��ڶ̹ܴ��ų��Թܢ��ڿ���������ֹˮ��ͨ���Թܢ��У����ž��Թܢ��ڿ�����Ȼ��ر�ֹˮ�У��Թܢ��ڲ�����H2���ݳ�����ѹ���Թܢ���FeSO4��Һ��ͨ��������ѹ���Թܢ��ڣ��ڴ�ѹҺ�����У�Һ�嶼����H2�������Ӷ������˿�����O2��������ʹ��Fe��OH��2�ܱ��ָ���ʱ�䡣


 �Ͻ�ƽСѧ��������ϵ�д�
�Ͻ�ƽСѧ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 33�����������ַ��������Ƶð�ɫ��Fe��OH��2������
33�����������ַ��������Ƶð�ɫ��Fe��OH��2�������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2011?��ɽ��ģ�����������ַ��������Ƶð�ɫ��Fe��OH��2������
��2011?��ɽ��ģ�����������ַ��������Ƶð�ɫ��Fe��OH��2�������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����һ���ò���Fe3+��FeSO4��Һ���ò���O2������ˮ���Ƶ�NaOH��Һ��Ӧ�Ʊ���
��1������������������������FeSO4��Һʱ������___________________________________��
��2����ȥ����ˮ���ܽ��O2������______________�ķ�����
��3�����ɰ�ɫFe(OH)2�����IJ������ó��ι���ȡ����O2��NaOH��Һ������FeSO4��ҺҺ���£��ټ���NaOH��Һ������������������______________________________________��
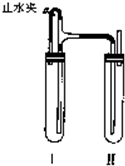
������������ͼװ���У���NaOH��Һ����м��ϡH2SO4���Լ��Ʊ���

��1�����Թܢ��������Լ���____________________________________��
��2�����Թܢ��������Լ���____________________________________��
��3��Ϊ���Ƶð�ɫFe(OH)2���������Թܢ�͢��м����Լ�����ֹˮ�У��������Ӻ��ʵ�鲽����___________________________________________________________________��
��4���������ɵ�Fe(OH)2�����ܽϳ�ʱ�䱣�ְ�ɫ����������_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������ַ��������Ƶð�ɫ��Fe(OH)2����������һ���ò���Fe3+��FeSO4��Һ���ò���O2������ˮ���Ƶ�NaOH��Һ��Ӧ�Ʊ�����1������������������������FeSO4��Һʱ�������________________________________��
��2����ȥ����ˮ���ܽ��O2�����õķ�����_______________________________________��
��3�����ɰ�ɫFe(OH)2�����IJ������ó��ι���ȡ����O2��NaOH��Һ������FeSO4��ҺҺ���£��ټ���NaOH��Һ������������������_____________________________________
_______________________________________________________________________________��
��������������ͼװ���У���NaOH��Һ����м��ϡH2SO4���Լ��Ʊ���
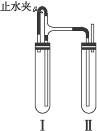
��1�����Թܢ��������Լ���___________________��
��2�����Թܢ��������Լ���___________________��
��3��Ϊ���Ƶð�ɫFe(OH)2���������Թܢ�͢��м����Լ�����ֹˮ�У��������Ӻ��ʵ�鲽����_______________________________________________________________________��
��4���������ɵ�Fe(OH)2�����ܽϳ�ʱ�䱣�ְ�ɫ����������________________________
_______________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�긣��ʡ������ѧ�ڵڶ����¿���ѧ�Ծ��������棩 ���ͣ������
��12�֣����������ַ��������Ƶð�ɫ��Fe(OH)2������
����һ���ò���Fe3����FeSO4��Һ�벻��O2������ˮ���Ƶ�NaOH��Һ��Ӧ�Ʊ���
������������������������FeSO4��Һʱ�������____________��
�Ƴ�ȥ����ˮ���ܽ��O2������________�ķ�����
�����ɰ�ɫFe(OH)2�����IJ������ó��ι���ȡ����O2��NaOH��Һ������FeSO4��ҺҺ���£��ټ���NaOH��Һ������������������______________________________��

������������ͼ��ʾװ���У���NaOH��Һ����м��ϡH2SO4���Լ��Ʊ���
�����Թܢ��������Լ���____________��
�����Թܢ��������Լ���____________��
���������ɵ�Fe(OH)2�����ܽϳ�ʱ�䱣�ְ�ɫ����������____________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com