
��11�֣���ͼ��ʾij��̬����A���仯����֮���ת����ϵ��ijЩ����ͷ�Ӧ��������ȥ��������A����ij��̬���ʻ������ɻ�����B��

��1����������B������������Ҫ��Ⱦ�������D����ɵ���AԪ�ص�����������Ӧˮ�������A�� ���������ƣ�����������Bͨ����ˮ�й۲쵽�������� ��������˵��������B����
�ԣ�д��һ���ɻ�����D����B�Ļ�ѧ����ʽ ��
��2����������B����������ӡˢ��·�壬������B��E�����Ԫ����ͬ��������C����ɵ���AԪ�ص�����������Ӧˮ�������B��������ˮ����ԭ���� �������ӷ���ʽ��ʾ����ͬ����ӡˢ��·�������з�������Ҫ��Ӧ�� ������������Һ���Ƿ��л�����B��ʵ����������� ��
��1���ǣ�[1��]����ˮ��ɫ������ɫ��dz��[1��]����ԭ[1��]��
C+2H2SO4��Ũ��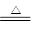 CO2��+2SO2��+2H2O[2��]����Cu��Na2SO3
+H2SO4��Ũ���ȴ𰸣�
CO2��+2SO2��+2H2O[2��]����Cu��Na2SO3
+H2SO4��Ũ���ȴ𰸣�
��2��Fe3++3H2O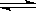 Fe(OH)3(����)+3H+[2��]��2Fe3++Cu=2Fe2++Cu2+[2��]��
Fe(OH)3(����)+3H+[2��]��2Fe3++Cu=2Fe2++Cu2+[2��]��
ȡ����[1��]������Һ���μ�KSCN��Һ[1��]���۲�����
����������1������������Ҫ��Ⱦ����SO2������B��SO2����A�ǵ���S��C����������D�����ᣬE�������������Ρ�SO2���л�ԭ�ԣ��ܱ���ˮ��������ʹ��ˮ��ɫ��Ũ�����ijЩ��ԭ�����ʷ�Ӧ���Ա���ԭ����SO2��
��2��������B����������ӡˢ��·�壬��B���Ȼ���������E���Ȼ�������A������C������������D�����������Ȼ�������Һ��ˮ�����������������壬���������ԣ�����ʽΪFe3++3H2O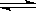 Fe(OH)3(����)+3H+���Ȼ�������������ͭ������ʽΪ2Fe3++Cu=2Fe2++Cu2+�����������ӿ���KSCN��Һ����ȡ����������Һ���μ�KSCN��Һ���۲�����
Fe(OH)3(����)+3H+���Ȼ�������������ͭ������ʽΪ2Fe3++Cu=2Fe2++Cu2+�����������ӿ���KSCN��Һ����ȡ����������Һ���μ�KSCN��Һ���۲�����


 ʱ�����������ҵԭ���ܳ�����ϵ�д�
ʱ�����������ҵԭ���ܳ�����ϵ�д� ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

��1��д��A�ڼ�����������H2��Ӧ�Ļ�ѧ����ʽ
��2��д��E��A���⻯�ﷴӦ����A�Ļ�ѧ����ʽ
��3��д��һ����D����B�Ļ�ѧ����ʽ ;
��4����5mL0.10mol��L-1��E��Һ��10mL0.10mol��L-1��NaOH��Һ��ϡ�
��д����Ӧ�����ӷ���ʽ ;
�ڷ�Ӧ����Һ��pH 7������ڡ�����С�ڡ����ڡ����������� ;
�ۼ��ȷ�Ӧ�����Һ����pH ���� �����������䡱��С������������
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾij��̬����A���仯����֮���ת����ϵ(ijЩ����ͷ�Ӧ��������ȥ)��������B�ڳ��³�ѹ��Ϊ���壬B��C����Է�������֮��Ϊ4��5��������D����Ҫ�Ĺ�ҵԭ�ϡ�
![]() ��1��д��A�ڼ�����������H2��Ӧ�Ļ�ѧ����ʽ
��1��д��A�ڼ�����������H2��Ӧ�Ļ�ѧ����ʽ
![]() _________________________________________________��
_________________________________________________��
![]() ��2��д��E��A���⻯�ﷴӦ����A�Ļ�ѧ����ʽ ��
��2��д��E��A���⻯�ﷴӦ����A�Ļ�ѧ����ʽ ��
![]() ��3��д��һ����D����B�Ļ�ѧ����ʽ ______________________��
��3��д��һ����D����B�Ļ�ѧ����ʽ ______________________��
![]() ��4����5mL0.10mol��L-1��E��Һ��10mL0.10 mol��L-1��NaOH��Һ��ϡ�
��4����5mL0.10mol��L-1��E��Һ��10mL0.10 mol��L-1��NaOH��Һ��ϡ�
![]() ��д����Ӧ�����ӷ���ʽ________________________��
��д����Ӧ�����ӷ���ʽ________________________��
![]() �ڷ�Ӧ����Һ��pH ______7(����ڡ�����С�ڡ����ڡ�)��������________��
�ڷ�Ӧ����Һ��pH ______7(����ڡ�����С�ڡ����ڡ�)��������________��
![]() �ۼ��ȷ�Ӧ�����Һ����pH________(����������䡱��С��)��������________________________________��
�ۼ��ȷ�Ӧ�����Һ����pH________(����������䡱��С��)��������________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2008����ͨ�ߵ�ѧУ����ͳһ���Ի�ѧ���⣨���Ͼ��� ���ͣ������
��ͼ��ʾij��̬����A���仯����֮���ת����ϵ(ijЩ����ͷ�Ӧ��������ȥ)��������B�ڳ��³�ѹ��Ϊ���壬B��C����Է�������֮��Ϊ4��5��������D����Ҫ�Ĺ�ҵԭ�ϡ�
(1)д��A�ڼ�����������H2��Ӧ�Ļ�ѧ����ʽ
____________________________________________________________
(2)д��E��A���⻯�ﷴӦ����A�Ļ�ѧ����ʽ___________________________
(3)д��һ����D����B�Ļ�ѧ����ʽ____________________________________��
(4)��5mL0.10mol��L��1��E��Һ��10mL0.10 mol��L��1��NaOH��Һ��ϡ�
��д����Ӧ�����ӷ���ʽ__________________________________________��
�ڷ�Ӧ����Һ��pH ______7(����ڡ�����С�ڡ����ڡ�)��������________��
�ۼ��ȷ�Ӧ�����Һ����pH________(����������䡱��С��)��������________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ������ʮУ�����������ѧ���ڳ����Ի�ѧ�Ծ����������� ���ͣ������
��11�֣���ͼ��ʾij��̬����A���仯����֮���ת����ϵ��ijЩ����ͷ�Ӧ��������ȥ��������A����ij��̬���ʻ������ɻ�����B��
��1����������B������������Ҫ��Ⱦ�������D����ɵ���AԪ�ص�����������Ӧˮ�������A�� ���������ƣ�����������Bͨ����ˮ�й۲쵽�������� ��������˵��������B����
�ԣ�д��һ���ɻ�����D����B�Ļ�ѧ����ʽ ��
��2����������B����������ӡˢ��·�壬������B��E�����Ԫ����ͬ��������C����ɵ���AԪ�ص�����������Ӧˮ�������B��������ˮ����ԭ���� �������ӷ���ʽ��ʾ����ͬ����ӡˢ��·�������з�������Ҫ��Ӧ�� ������������Һ���Ƿ��л�����B��ʵ����������� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com