
Mͨ�����й�ϵ���Եõ���˾ƥ��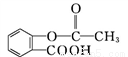 ���ܷų�����Ϣʹ(
���ܷų�����Ϣʹ(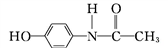 )�ĸ߷���ҩ��(
)�ĸ߷���ҩ��(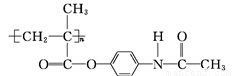 )��
)��
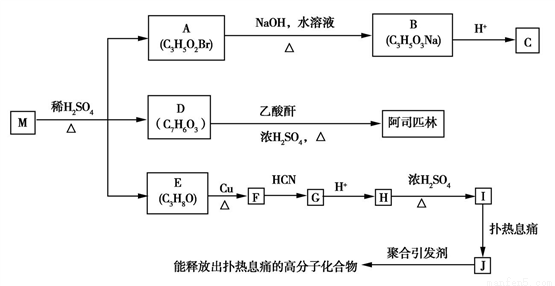
��֪:��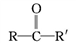
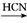

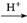
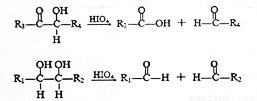
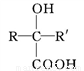 (R��R'��ʾ��ԭ�ӻ�����)��
(R��R'��ʾ��ԭ�ӻ�����)��
��C�к���һ����������H��Ϊͬϵ�
��ش�����:
(1)I���еĹ����ŵ�����Ϊ________��
(2)F��G�ķ�Ӧ����Ϊ________��I��J�ķ�Ӧ����Ϊ_______��
(3)M�Ľṹ��ʽΪ________��
(4)��������������D��ͬ���칹����____�֡�
������FeCl3��Һ������ɫ��Ӧ�����ܷ���������Ӧ
(5)��E��F�Ļ�ѧ����ʽΪ_______________________��
��1 mol��˾ƥ��������NaOH��Һ��Ӧ�������NaOH�����ʵ���Ϊ________mol����˾ƥ����������NaOH��Һ���ȵĻ�ѧ����ʽΪ:_________________��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017��ӱ�ʡ������ѧ�ڵڶ����ʼ쿼�������ۺϻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
����������ӷ���ʽ��д��ȷ���ǣ� ��
A. NH4HSO3��Һ������������������Һ��ϼ��ȣ�NH4++OH-=NH3��+H2O
B. ��NaHCO3��Һ�м�������ij���ʯ��ˮ��Ca2++2OH-+2HCO3-=CaCO3��+2H2O+ CO32-
C. �����ʵ�����FeBr2��Cl2����Һ�еķ�Ӧ��2Fe2++2Br-+2Cl2=2Fe3++Br2+4Cl-
D. ��84����Һ���͡�����顱����Ҫ�ɷ�Ϊ���ᣩ���ʹ�û�����ж����壺ClO3-+5Cl-+6H+=3Cl2��+3H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ɽ��ʡ��ׯ�б�У����һ��ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
���������У���ȷ����
A. C��N��OԪ�صĵ�������������Խ��Խ��
B. Li��Na��K���������ˮ����ļ������μ���
C. Na��Mg��Al�ļ����ӵ���������������ǿ
D. P��S��ClԪ�ص���������������ߣ����Ӧ����̬�⻯����ȶ������μ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�찲��ʡ�����и����ڶ���ģ�⿼�������ۺϻ�ѧ�Ծ��������棩 ���ͣ��ƶ���
��֬������Ӫ�����ʺ���Ҫʳ�Ҳ��һ����Ҫ����ҵԭ�ϡ�����������֬Ϊ��Ҫԭ�ϻ�ò��ֲ�Ʒ�ĺϳ�·�ߣ�
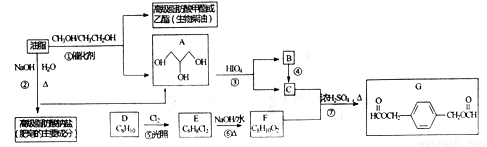
��֪��
��R1��R2��R3��R4�������⡢�������������ţ�
�ش��������⣺
��1�����й�����֬��˵����ȷ����____________. �����ţ�
a����֬����ֲ���ͺ�֬������������
b.��Ȼ��֬�ǻ����̶����۵�ͷе�
c����֬����Ȼ�߷��ӻ�����
d��Ӳ�����ֽ�����֬�������ڴ�������䣬�����ױ�������������
��2��G�й����ŵ�����Ϊ________����Ӧ�ٵķ�Ӧ����Ϊ__________
��3����ϵͳ������д��A������__________, C��F��Ӧ����G�Ļ�ѧ����ʽΪ_____��
��4����Ԫȡ�����㻯����H��G��ͬ���칹�壬H��������������
�� �ܷ���������Ӧ���� ����������ˮ��������ʵ���֮��Ϊ2: 1���� ����NaHCO3��Һ��Ӧ��
���������������H����_____�֣�����������ṹ��������G�����������к˴Ź�������Ϊ�����Ľṹ��ʽΪ_________________��д��һ�ּ��ɣ���
��5��д����HOCH2CH2OH HCOOCH2CH2OOCH �ĺϳ�·�ߣ����Լ���ѡ���ϳ�·�߲������е���д��ʽ��________________��
HCOOCH2CH2OOCH �ĺϳ�·�ߣ����Լ���ѡ���ϳ�·�߲������е���д��ʽ��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�찲��ʡ�����и����ڶ���ģ�⿼�������ۺϻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
��һ��ȫ��������ӵ��ʹ������������ʣ����ñ����Ĥ�����ͼ�ѹ���ͼ���������˲��Ͽ���������Ӵ����ԣ��Ӷ�ʵ���������е��ѹ�ij�ŵ硣��صĹ���ԭ��Ϊ��LiMO2+nC Li1-xMO2+LixCn (M������Co��Ni��Fe��)���ڲ��ṹ��ͼ��ʾ�������й�˵����ȷ����
Li1-xMO2+LixCn (M������Co��Ni��Fe��)���ڲ��ṹ��ͼ��ʾ�������й�˵����ȷ����

A. ��طŵ�ʱ�����������ķ�ӦΪ��nC+xLi+��xe-��LixCn
B. �������a �˽ӵ�Դ�ĸ���,b�˽ӵ�Դ������
C. ��س��ʱLi+���������ƶ�
D. ��ع���ʱ���������������ڶ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ɹų���������ظ���һģ�����ۺϻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����£���100mL0.1mol/LNH4HSO4��Һ�еμ�0.1mol/L��NaOH��Һ��������ҺPH��NaOH��Һ����Ĺ�ϵ������ͼ��ʾ������˵����ȷ����

A. a��b��c��d�ĸ����У�ˮ�ĵ���̶�������d
B. a����Һ�У�c(NH3��H2O)+c(H+)=c(OH��)
C. b����Һ�У�c(Na+)+c(NH4+)=2c(SO42��)
D. c����Һ�У�c(Na+)= c(SO42��) +c(NH4+)+ c(NH3��H2O)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�����ʡ����2��ģ�������ۺϻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
�췯�ƣ��ظ����ƣ� ������Ҫ�Ļ�������ԭ�ϣ�Ӧ��ʮ�ֹ㷺����ҵ�Ʊ��췯�Ƶ��������£�
������Ҫ�Ļ�������ԭ�ϣ�Ӧ��ʮ�ֹ㷺����ҵ�Ʊ��췯�Ƶ��������£�
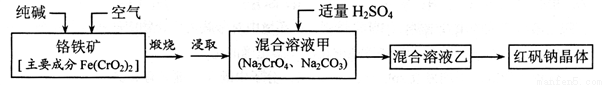
��ش��������⣺
(1) �Ļ�̬�����Ų�ʽΪ____________��
�Ļ�̬�����Ų�ʽΪ____________��
(2)���ո���������Ӧ��____________ ��ƽ������ѧ��Ӧ����ʽ��
��ƽ������ѧ��Ӧ����ʽ��
(3)��Һ���м���H2SO4ʱ�����Թ۲쵽��Һ�ɻ�ɫ��Ϊ��ɫ�����ų���ɫ���壬�� �ط�Ӧ�����ӷ���ʽΪ____________��
(4)�Ʊ��췯�Ƶķ�ˮ������д����������ữ��ˮ������+6�۸������Է�Һ��������ڣ��������������е�⣬��������H2,�������ﱻ ����Ȼ��ת��ΪCr(OH)3�� Fe(0H)3 ������
����Ȼ��ת��ΪCr(OH)3�� Fe(0H)3 ������
�������ĵ缫��ӦʽΪ�������Ǻ�����Ӧ)��____________��
����Һ�н����������������ɳ�����ȫ����ˮ�ĵ���ƽ��ǶȽ�����ԭ��______��
����֪ij�� �����Է�ˮ��CrԪ�صĺ�����52.5mg/L,������CrԪ��������� ����Ϊ0.5 mg/L��Ҫ����100 m3��ˮ�ﵽ�ŷű�������������_____kg��
�����Է�ˮ��CrԪ�صĺ�����52.5mg/L,������CrԪ��������� ����Ϊ0.5 mg/L��Ҫ����100 m3��ˮ�ﵽ�ŷű�������������_____kg��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�߶�2��������ѧ�Ծ��������棩 ���ͣ�ѡ����
���ʵ���Ũ����ͬ��������Һ����Na2CO3 ��NaHCO3 ��H2CO3 ��(NH4)2CO3 ��NH4HCO3����c(CO32-)��С��������˳����ȷ����( )
A����<��<��<��<�� B����<��<��<��<��
C����<��<��<��<�� D����<��<��<��<��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017���Ĵ�ʡ�ɶ��и����ڶ���ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
ij��ѧС����ͨ��ʵ��̽����I.����Һ�巢��ȡ����Ӧ����.����Һ���ڴ��������µķ�Ӧ����������װ�D��ͼ��ʾ��
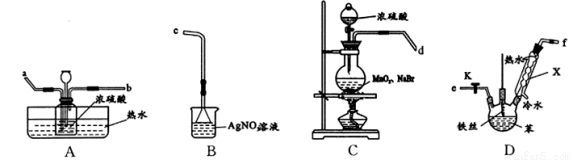
��֪��MnO2+2NaBr+2H2SO4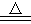 Br2��+MnSO4+Na2SO4+2H2O
Br2��+MnSO4+Na2SO4+2H2O
��1��ʵ�����Ʊ��屽�Ļ�ѧ����ʽΪ___________________������X������Ϊ___________��
��2����ȡ�屽��ʵ������������£�
������װ�D����ӿ�˳��Ϊ������ĸ����d��____�� _____��_____�� ______��c��
�ڼ��װ�������ԣ�
��C�м������ҩƷ��_______���ٴ�D������K����ȼC���ƾ��ƣ�������˿�ڻ��Һ�У�һ��ʱ���D��Һ����ڣ�ԭ����_________ ��
�ܹر�C�л�����
��3��A�жԹ��ƿ������ˮԡ��Ŀ���� _______________��
��4����B���е���ɫ�������ɣ��ܷ�ȷ������Һ�巢����ȡ����Ӧ��_____(���ܡ���)��
��5���������ף�����Һ����FeBr3���µķ�Ӧ��������������
I Br2+FeBr3 Br++FeBr4-
Br++FeBr4-
��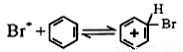
������ɵ�������Ӧ����__________________________��
�ڸ�С�齫Aװ����Ũ�����滻��ϡ����ʱʵ��ʧ�ܣ��Դӷ�Ӧ�����Ʋ����ԭ����__________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com