
��һ��ɫ��Һ�����ܺ���Fe3+��Al3+��Fe2+��Mg2+��Cu2+��NH4+��K+��CO32-��SO42-�����ӵļ��֣�Ϊ������ɷ֣�ȡ����Һ�ֱ�������ĸ�ʵ�飬��������й���������ͼ��ʾ��

�ڵڢ۸�ʵ���У����ɰ�ɫ�������������NaOH��������ͼ��ʾ�����ϵ���ݴ˿�֪��

����ԭ��Һ��һ�������ڵ�������_____________________��
��Ϊ�������Һ��һ�����ڵ����ӵ�Ҫ��һ����ܽ���
�ֳ�������Ϊ��д��ѧʽ��___________��_________��
��д���ڢ۸�ʵ���з�����Ӧ�����ӷ���ʽ
��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��8�֣���һ��ɫ��Һ�����п��ܺ���Fe3+��Al3+��Fe2+��Mg2+��Cu2+��NH4+��K+��CO32����SO42�������ӵļ��֣�Ϊ������ɷ֣�ȡ����Һ�ֱ�������ĸ�ʵ�飬��������й���������ͼ��ʾ��
��1����ԭ��Һ��һ�������ڵ������� ��
��2���ڢ۸�ʵ���У���ȡԭ��Һ�����Ϊ100 mL���μӵ�NaOH��Һ�����ʵ���Ũ��Ϊ0.5 mol��L��1 �����ɰ�ɫ�������������NaOH��������ͼ��ʾ�����ϵ�������Һ�����������ӵ����ʵ���Ũ��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ӱ�ʡε��һ�и�һ��ѧ����ĩ���Ի�ѧ���� ���ͣ������
��8�֣���һ��ɫ��Һ�����п��ܺ���Fe3+��Al3+��Fe2+��Mg2+��Cu2+��NH4+��K+��CO32����SO42�������ӵļ��֣�Ϊ������ɷ֣�ȡ����Һ�ֱ�������ĸ�ʵ�飬��������й���������ͼ��ʾ��






 ��1����ԭ��Һ��һ�������ڵ������� ��
��1����ԭ��Һ��һ�������ڵ������� ��
��2���ڢ۸�ʵ���У���ȡԭ��Һ�����Ϊ100 mL���μӵ�NaOH��Һ�����ʵ���Ũ��Ϊ0.5 mol��L��1�����ɰ�ɫ�������������NaOH��������ͼ��ʾ�����ϵ�������Һ�����������ӵ����ʵ���Ũ��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������ʡμ���и����ڶ���������⻯ѧ�Ծ��������棩 ���ͣ������
��10�֣���һ����Һ�����ܺ��� �������е�һ�ֻ��֡��ּ���Na2O2��ĩֻ����ɫ��ζ������ų�����ͬʱ������ɫ�����������Na2O2���������ɰ�ɫ��������֮��Ĺ�ϵ����ͼ����ʾ��
�������е�һ�ֻ��֡��ּ���Na2O2��ĩֻ����ɫ��ζ������ų�����ͬʱ������ɫ�����������Na2O2���������ɰ�ɫ��������֮��Ĺ�ϵ����ͼ����ʾ��
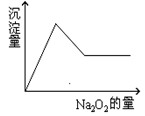
���ƶϣ�ԭ��Һ��һ������ ��һ��û�� �����ܺ� ��Ϊ�˽�һ��ȷ�����ܺ��е����� ��Ӧ���ӵ�ʵ�����Ϊ ������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ӱ�ʡ��һ��ѧ����ĩ���Ի�ѧ���� ���ͣ������
��8�֣���һ��ɫ��Һ�����п��ܺ���Fe3+��Al3+��Fe2+��Mg2+��Cu2+��NH4+��K+��CO32����SO42�������ӵļ��֣�Ϊ������ɷ֣�ȡ����Һ�ֱ�������ĸ�ʵ�飬��������й���������ͼ��ʾ��


 ��1����ԭ��Һ��һ�������ڵ�������
��
��1����ԭ��Һ��һ�������ڵ�������
��
��2���ڢ۸�ʵ���У���ȡԭ��Һ�����Ϊ100 mL���μӵ�NaOH��Һ�����ʵ���Ũ��Ϊ0.5 mol��L��1 �����ɰ�ɫ�������������NaOH��������ͼ��ʾ�����ϵ�������Һ�����������ӵ����ʵ���Ũ��Ϊ ��

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com