
±�������л��ϳɵ���Ҫ�м��壬ij��ѧ��ȤС���ͬѧ�������Ϸ��֣�
����l��������ŨH2SO4���廯�ƻ��������Ʊ�1���嶡�飮
������Ӧ��CH3CH2CH2CH2OH��HBr![]() CH3CH2CH2CH2Br��H2O��
CH3CH2CH2CH2Br��H2O��
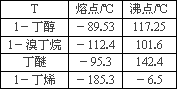
(1)�Ʊ�1���嶡���װ��Ӧѡ����ͼ�е�_________(�����)����Ӧ����ʱ���¶Ȳ��˳���100�棬������_________��
(2)�Ʊ������У������Ũ������廯�Ƶ�������_________��
(3)��Ӧ��������Ӧ�������1���嶡����������Ӧѡ�õ�װ����_________���ò���Ӧ���Ƶ��¶�(t)��Χ��_________��
(4)����ȥ������е���������Br2���������������ʺϵ���_________��(����ĸ)
A��NaI
B��NaOH
C��NaHSO3
D��KCl
 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ˮ�и�����Ԫ�ء�
��1��KCl�㷺Ӧ����ҽҩ��ũҵ��KCl�����л�̬�������ӵ����Ų�ʽΪ
��2�������Dz��ֽ���Ԫ�صĵ�����
| X | Y | Z | |
| ��һ������(KJ/mol) | 520.2 | 495.8 | 418.8 |
��֪X Y Z �ļ۲���ӹ���ΪnS1,�����ֽ������Ȼ��RCl�����۵��ɸߵ��͵�˳��Ϊ
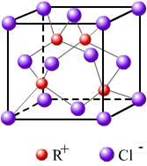
(3)RCl�����л����ϳɴ���, ����������, �����ȹ�ҵ��R�������е������ó���K��L��M �������Ӳ㣬����Cl���γɵľ���ṹ��ͼ��ʾ��R��Ԫ�ط����� ����ͬһ��Cl�������� R���� ����
(4) ±�������л��ϳ������þ������Ľṹ��±�����Ļ����кܴ��Ӱ�졣CH3��CH2��Cl�ͼ���Һ������ȡ����Ӧ����CH2��CH2��Cl �ͼ���Һ�������ã���ӽṹ�Ͻ��������
(5) HCl�� HF�ṹ���ƣ���������Ĵ���ʹ���������ϴ��ڽϴ���죬���оٳ����������Ӱ�쵼�µ����ʲ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���˽̰���л�ѧѡ��5 5.2Ӧ�ù㷺�ĸ߷��Ӳ�����ϰ���������棩 ���ͣ������
±�������л���ϳɵ�ħ��ʦ��±������±��ԭ�Ӻ�������ý��������ӽ�ϣ�����ȡ����Ӧ����
R��X��NaCN R��CN��NaX��
R��CN��NaX��
R��X��2Na��X��R�� R��R�䣫2NaX.
R��R�䣫2NaX.
G���ܵ���ľۺ���������и����ʵ�ת����ϵ�ش�

(1)A�ķ���ʽ________��E�Ľṹ��ʽ___________________________________��
(2)д��B��D�Ļ�ѧ����ʽ��_____________________________________________
________________________________________________________________________.
(3)д��C2H3Cl�D��C�Ļ�ѧ����ʽ��_____________________________________
________________________________________________________________________.
(4)д��A�D��G�Ļ�ѧ����ʽ��_________________________________________
________________________________________________________________________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ������
 ��һ�ֳ����Ļ�ױƷ��ù�������������������£���Ӧ����û��ȫ��ע������
��һ�ֳ����Ļ�ױƷ��ù�������������������£���Ӧ����û��ȫ��ע������ 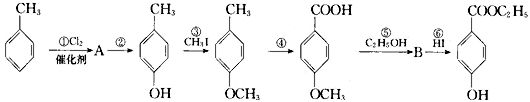
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com