
����������Ч����SO2�Կ�������Ⱦ��
(1)��ú�м���ʯ��ʯ�ɼ���ȼ�ղ�����SO2�ĺ������÷�Ӧ�Ļ�ѧ����ʽ��____________________________ ��
(2)��ˮ�������ԣ���Ҫ����Na����K����Ca2����Mg2����Cl����SO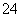 ��Br����HCO
��Br����HCO �ȡ���SO2�����������ú�ˮ�����乤��������ͼ��ʾ��
�ȡ���SO2�����������ú�ˮ�����乤��������ͼ��ʾ��

������������ͨ�������Ŀ����______________________________________________��
��ͨ��������������еĺ�ˮ����Ȼ��ˮ��ȣ�Ũ�������Բ�ͬ��������______(����ĸ)��
a��Cl�� b��SO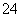 c��Br�� d��HCO
c��Br�� d��HCO
(3)��NaOH��Һ���������е�SO2�������õ�Na2SO3��Һ���е�⣬�ɵõ�NaOH��ͬʱ�õ�H2SO4����ԭ����ͼ��ʾ��(�缫����Ϊʯī)

��ͼ��a�����ӵ�Դ��______(���������)����C��������������________��
��SO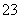 �ŵ�ĵ缫��ӦΪ________________________��
�ŵ�ĵ缫��ӦΪ________________________��
�۵�����������������������ǿ����ƽ���ƶ���ԭ�����ͼ�����ǿ��ԭ��________________________________________________________________________��
�𰸡�(1)2SO2��O2��2CaCO3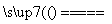 2CaSO4��2CO2
2CaSO4��2CO2
(2)�ٽ�H2SO3��HSO ������ΪSO
������ΪSO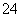 ����bd
����bd
(3)�ٸ������ᡡ��SO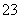 ��2e����H2O===SO
��2e����H2O===SO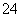 ��2H��
��2H��
��H2O??H����OH����H���������ŵ�����H2��c(H��)��С��ˮ�ĵ���ƽ�������ƶ���������ǿ
������(1)úȼ��ʱ��ʯ��ʯ�ڸ����·ֽ����CaO��CO2��CaOΪ���������������SO2��O2��Ӧ����CaSO4���÷�Ӧ�Ļ�ѧ����ʽ��2SO2��O2��2CaCO3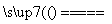 2CaSO4��2CO2��(2)SO2Ϊ�����������ˮ�������ԣ���������ͨ�������Ŀ���ǽ�SO
2CaSO4��2CO2��(2)SO2Ϊ�����������ˮ�������ԣ���������ͨ�������Ŀ���ǽ�SO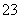 ��HSO
��HSO ��������ͨ���������Һ��SO
��������ͨ���������Һ��SO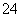 ��Ũ������HCO
��Ũ������HCO ��Ũ�ȼ�С��(3)���Na2SO3��Һ������ͼʾ��a�����ӵ�Դ�ĸ�����C�����������������ᡣ�����ĵ缫��ӦʽΪ2H����2e��===H2���������ĵ缫��ӦʽΪSO
��Ũ�ȼ�С��(3)���Na2SO3��Һ������ͼʾ��a�����ӵ�Դ�ĸ�����C�����������������ᡣ�����ĵ缫��ӦʽΪ2H����2e��===H2���������ĵ缫��ӦʽΪSO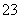 ��2e����H2O===SO
��2e����H2O===SO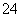 ��2H������������H���������ŵ�����H2����������
��2H������������H���������ŵ�����H2����������
��ƽ��H2O??H����OH����c(H��)��С��ˮ�ĵ���ƽ�������ƶ���������ǿ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и�ԭ�ӻ����ӵĵ����Ų�ʽ�������(����)
A��C��1s22s22p2
B��O2����1s22s22p6
C��Cr��1s22s22p63s23p63d44s2
D��Al3����1s22s22p6
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ŵ������ѧ��������ά���о����֣���������������NaIʱ��Na����I�����˼����10��15 �������Ӽ��������˿���Լ2.8
�������Ӽ��������˿���Լ2.8 ʱ���ʹ��ۼ���������ά�����о��ɹ��ܵó��Ľ�����(����)
ʱ���ʹ��ۼ���������ά�����о��ɹ��ܵó��Ľ�����(����)
A��NaI���������Ӿ���ͷ��Ӿ���Ļ���� B�����ۼ������Ӽ�û�����ԵĽ���
C��NaI�����м������Ӽ������й��ۼ� D�����Ӿ�����ܺ��й��ۼ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)���������У����γ��������______��
A���������� B�����ȴ���
C��������̼ D������
(2)�������¼��ִ�ʩ���ٶ�ȼ��úʱ������β�����г�������������ԭú��ȼ�ϣ���ȼúʱ���������������ܿ��������Դ�������ܼ�����������Ĵ�ʩ��______��
A���٢ڢ� B���ڢۢ�
C���٢ڢ� D���٢ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ij������Һ(���ܺ���Br����SO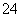 ��H2SO3��NH
��H2SO3��NH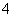 )�ֱ��������ʵ�飺
)�ֱ��������ʵ�飺
�ټ���ʱ�ų����������ʹƷ����Һ��ɫ��
�ڼӼ�������Ժ���ʱ�ų����������ʹ��ʪ�ĺ�ɫʯ����ֽ������
�ۼ�����ˮʱ����Һ���Ի�ɫ���ټ���BaCl2��Һ�������İ�ɫ����������ϡ���ᡣ
�����������ʲ���ȷ������ԭ��Һ���Ƿ���ڵ���(����)
A��Br�� B��SO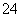 C��H2SO3 D��NH
C��H2SO3 D��NH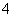
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������(Na2S2O5)�dz��õ�ʳƷ��������֮һ��ij�о�С���������ʵ�飺
ʵ��һ�����������Ƶ���ȡ
������ͼװ��(ʵ��ǰ�ѳ���װ���ڵĿ���)��ȡNa2S2O5��װ�â�����Na2S2O5���������������ķ�ӦΪNa2SO3��SO2===Na2S2O5��
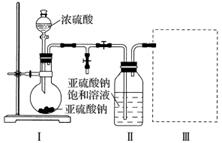
(1)װ�â��в�������Ļ�ѧ����ʽΪ__________________________________________��
(2)Ҫ��װ�â��л���������ľ��壬�ɲ�ȡ�ķ��뷽����________________________________________________________________________
________________________________________________________________________��
(3)װ�â����ڴ���β������ѡ�õ������װ��(�г���������ȥ)Ϊ__________(�����)��
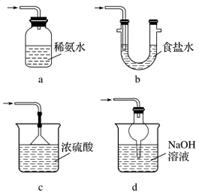
ʵ��������������Ƶ�����
Na2S2O5����ˮ������NaHSO3��
(4)֤��NaHSO3��Һ��HSO �ĵ���̶ȴ���ˮ��̶ȣ��ɲ��õ�ʵ�鷽����_____(�����)��
�ĵ���̶ȴ���ˮ��̶ȣ��ɲ��õ�ʵ�鷽����_____(�����)��
a���ⶨ��Һ��pH
b������Ba(OH)2��Һ
c����������
d������Ʒ����Һ
e������ɫʯ����ֽ���
(5)����Na2S2O5�����ڿ������ѱ�������ʵ�鷽����__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������������������ȷ���������ϵ����(����)
| ѡ�� | ������ | ������ |
| A | H2�л�ԭ�ԣ�Ũ������ǿ������ | ������Ũ�������H2 |
| B | CuS������ˮ������ | ��Ӧ��H2S��CuSO4===CuS����H2SO4���Է��� |
| C | ŨH2SO4����ˮ�� | ŨH2SO4�����ڸ��ﰱ�� |
| D | SO2�������Ժ�Ư���� | ����ɫʯ����Һ��ͨ��SO2����Һ�ȱ������ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼΪ�������ӵĽṹʾ��ͼ�����������ա�
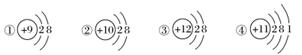
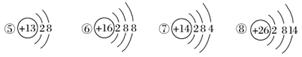
(1)���������ӽṹ��������________(���ţ���ͬ)��
(2)�����ȶ��Խṹ��ԭ����__________________��
(3)ֻ�ܵõ��ӵ�������______________��ֻ��ʧ���ӵ�������______________�����ܵõ��ӣ�����ʧ���ӵ�������____________________________��
(4)�����Ӱ뾶________�����Ӱ뾶(����ڡ�����С�ڡ����ڡ�)��
(5)ijԪ��R�γɵ�������ΪR2O3����R�����ӽṹʾ��ͼ������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ͭ��һ����Ũ���ᷴӦ���õ�����ͭ��Һ��NO2��N2O4��NO�Ļ�����壬��Щ������5.6 L O2(��״��)��Ϻ�ͨ��ˮ�У�����������ȫ��ˮ�����������ᡣ������ͭ������Ϊ(����)
A��16 g B��32 g
C��64 g D��������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com