
ʵ���ҴӺ����Һ(��H2O�⣬����CCl4��I2��I����)�л��յ⣬��ʵ��������£�
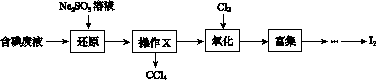
(1)���Һ�м����Թ�����Na2SO3��Һ������Һ�е�I2��ԭΪI���������ӷ���ʽΪ__________________���ò�����I2��ԭΪI����Ŀ����______________________��
(2)����X������Ϊ________��
(3)����ʱ����������ƿ�н���I����ˮ��Һ���������pHԼΪ2������ͨ��Cl2����40 �����ҷ�Ӧ(ʵ��װ����ͼ��ʾ)��
ʵ������ڽϵ��¶��½��е�ԭ����______________����ƿ��ʢ�ŵ���ҺΪ________��

(4)��֪��5SO ��2IO
��2IO ��2H��===I2��5SO
��2H��===I2��5SO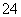 ��H2O
��H2O
ij�����ˮ(pHԼΪ8)��һ������I2�����ܴ���I����IO �е�һ�ֻ����֡��벹���������麬���ˮ���Ƿ���I����IO
�е�һ�ֻ����֡��벹���������麬���ˮ���Ƿ���I����IO ��ʵ�鷽����ȡ���������ˮ��CCl4�����ȡ����Һ��ֱ��ˮ���õ�����Һ���鲻���еⵥ�ʴ��ڣ�________________________________________________________________________
��ʵ�鷽����ȡ���������ˮ��CCl4�����ȡ����Һ��ֱ��ˮ���õ�����Һ���鲻���еⵥ�ʴ��ڣ�________________________________________________________________________
________________________________________________________________________
________________________________________________________________________��
ʵ���пɹ�ѡ����Լ���ϡ���ᡢ������Һ��FeCl3��Һ��Na2SO3��Һ��
(1)SO ��I2��H2O===2I����SO
��I2��H2O===2I����SO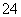 ��2H����ʹCCl4�еĵ����ˮ��
��2H����ʹCCl4�еĵ����ˮ��
(2)��Һ
(3)ʹ��������Һ���нϴ���ܽ��(���ֹI2�������ֹI2��һ��������)��NaOH��Һ
(4)��ˮ��ȡ������Һ������1��2 mL������Һ���������ữ���μ�FeCl3��Һ������Һ������˵����ˮ�к���I��������Һ��������˵����ˮ�в�����I��������ˮ��ȡ������Һ������1��2 mL������Һ���������ữ���μ�Na2SO3��Һ������Һ������˵����ˮ�к���IO ������Һ��������˵����ˮ�в�����IO
������Һ��������˵����ˮ�в�����IO
[����] (1)SO ��I2����ΪSO
��I2����ΪSO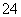 ��I2����ԭΪI������ϵ���غ��ԭ���غ�ɵ�SO
��I2����ԭΪI������ϵ���غ��ԭ���غ�ɵ�SO ��I2��H2O===2I����SO
��I2��H2O===2I����SO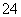 ��2H������ΪI2������ˮ�����⻯��������ˮ���ʽ�I2��ԭΪI����Ŀ����ʹ��Ԫ�ؽ���ˮ�㡣(2)�����л��ܼ���ˮ��Һ�Ļ������Ҫ��Һ��(3)�¶�Խ�ߣ�Cl2�ܽ��ԽС���������¶����ߣ�Cl2���I2��һ������ΪIO
��2H������ΪI2������ˮ�����⻯��������ˮ���ʽ�I2��ԭΪI����Ŀ����ʹ��Ԫ�ؽ���ˮ�㡣(2)�����л��ܼ���ˮ��Һ�Ļ������Ҫ��Һ��(3)�¶�Խ�ߣ�Cl2�ܽ��ԽС���������¶����ߣ�Cl2���I2��һ������ΪIO ����������I����Ч��ƫ�ͣ����⣬I2Ҳ����������
����������I����Ч��ƫ�ͣ����⣬I2Ҳ����������
(4)����I2�õ��ۣ���������Լ������ʣ�FeCl3���������ԣ��ɽ�I������ΪI2����Na2SO3����ǿ��ԭ�ԣ��ɽ�IO ��ԭΪI2��
��ԭΪI2��


 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й���Һ��ɵ�������������(����)
A����ɫ��Һ�п��ܴ�������Al3����NH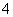 ��Cl����S2��
��Cl����S2��
B��������Һ�п��ܴ�������Na����ClO����SO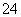 ��I��
��I��
C����������Һ�п��ܴ�������Na����K����Cl����HCO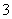
D��������Һ�п��ܴ�������Fe3����K����Cl����SO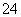
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪�� ���£�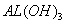 ��K�����ܽ��Զ����
��K�����ܽ��Զ����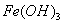 ����Ũ�Ⱦ�Ϊ0.1
����Ũ�Ⱦ�Ϊ0.1

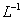 ��
��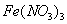 ��
��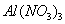 �����Һ�У���μ���NaOH ��Һ������ʾ��ͼ��ʾ����
�����Һ�У���μ���NaOH ��Һ������ʾ��ͼ��ʾ����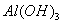 �����ʵ��������NaOH��Һ������Ĺ�ϵ ����������
�����ʵ��������NaOH��Һ������Ĺ�ϵ ����������
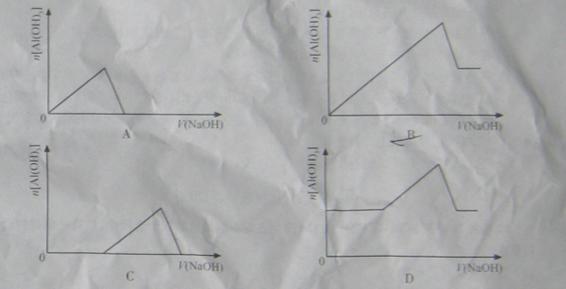
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʯī�ڲ�����������ҪӦ�á�ij����ʯī�к�SiO2(7.8%)��Al2O3(5.1%)��Fe2O3(3.1%)��MgO(0.5%)�����ʡ���Ƶ��ᴿ���ۺ����ù������£�
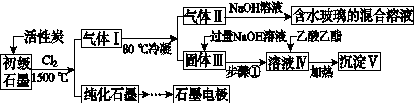
(ע��SiCl4�ķе�Ϊ57.6 �棬�����Ȼ���ķе������150 ��)
(1)��Ӧ����ͨ��Cl2ǰ����ͨһ��ʱ��N2����ҪĿ����____________________��
(2)���·�Ӧ��ʯī�����������ʾ�ת��Ϊ��Ӧ���Ȼ��������е�̼��������ҪΪ________�����������ij��õ�ˮ�����Ļ�ѧ��Ӧ����ʽΪ____________________________________________��
(3)�����Ϊ�����衢________��������Һ���е���������________��
(4)����Һ�����ɳ��������ܷ�Ӧ�����ӷ���ʽΪ______________________________________________��100 kg����ʯī�����ܻ�â�������Ϊ______kg��
(5)ʯī��������Ȼˮ����ͭ���ĵ绯ѧ�����������ͼ����ʾ��ͼ��������Ӧ��ע��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
LiPF6������ӵ���й㷺Ӧ�õĵ���ʡ�ij������LiF��PCl5Ϊԭ�ϣ����·�Ӧ�Ʊ�LiPF6�����������£�
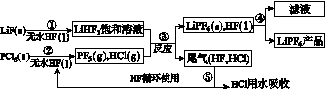
��֪��HCl�ķе��ǣ�85.0 �棬HF�ķе���19.5 �档
(1)�ڢٲ���Ӧ����ˮHF��������________________��________________����Ӧ�豸�����ò������ʵ�ԭ����______________________________________________(�û�ѧ����ʽ��ʾ)����ˮHF�и�ʴ�ԺͶ��ԣ�������ȫ�ֲ���ʾ�������С�Ľ�HFմ��Ƥ���ϣ���������2%��________��Һ��ϴ��
(2)������������ˮ�����½��У��ڢ۲���Ӧ��PF5����ˮ�⣬�����Ϊ�����ᣬд��PF5ˮ��Ļ�ѧ����ʽ��____________________________________��
(3)�ڢܲ�������õķ�����________���ڢݲ�����β����HF��HCl���õķ�����________��
(4)LiPF6��Ʒ��ͨ����������LiF��ȡ��Ʒw g�����Li�����ʵ���Ϊn mol�������Ʒ��LiPF6�����ʵ���Ϊ________mol(�ú�w��n�Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������H��He��N��Na��Mg��Si��Ԫ�أ�������δ������Դ���⡣
(1)3He�Ǹ�Ч����ԭ�ϣ���ԭ�Ӻ���������Ϊ________��
(2)Na��ԭ�ӽṹʾ��ͼΪ________��Na����������ȫȼ�����ò���ĵ���ʽΪ________��
(3)MgCl�ڹ�ҵ��Ӧ�ù㷺������MgO�Ʊ���
��MgO���۵��BaO���۵�________(��ߡ��͡�)��
��������ij��ʯ�������õ���MgO�к�������SiO2����ȥSiO2�����ӷ���ʽΪ__________________________��SiO2�ľ�������Ϊ________��
��MgO��̿�ۺ�������һ�������·�Ӧ���Ʊ�MgCl2����β����������NaOH��Һ��ȫ���գ������ɵ���Ϊ________________(д��ѧʽ)��
(4)�����к��зḻ��3He��������������1 kg 3He��ͬʱ�ɵ�6000 kg H2��700 kg N2����������H2��N2Ϊԭ�Ͼ�һϵ�з�Ӧ��������̼�����________kg��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
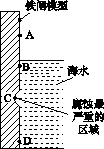
�����仯�����������������ϵ���С�
(1)��ͼ��ʵ�����о���ˮ����բ��ͬ��λ��ʴ���������ʾ��ͼ��
�ٸõ绯��ʴ��Ϊ________��
��ͼ��A��B��C��D�ĸ�������������������________(����ĸ)��
(2)�÷���Ƥ��ȡ����(Fe2O3)�IJ�������ʾ��ͼ���£�
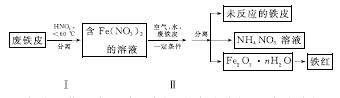
�ٲ�������¶ȹ��ߣ�����������ֽ⡣����ֽ�Ļ�ѧ����ʽΪ______________________________��
�ڲ�����з�����Ӧ��4Fe(NO3)2��O2��(2n��4)H2O===2Fe2O3��nH2O��8HNO3����Ӧ������HNO3�ֽ�����Ƥ�е���ת��ΪFe(NO3)2���÷�Ӧ�Ļ�ѧ����ʽΪ____________________________��
���������������У������֡���ɫ��ѧ��˼�����______(��дһ��)��
(3)��֪t ��ʱ����ӦFeO(s)��CO(g)Fe(s)��CO2(g)��ƽ�ⳣ��K��0.25��
��t ��ʱ����Ӧ�ﵽƽ��ʱn(CO)��n(CO2)��________��
������1 L�ܱ������м���0.02 mol FeO(s)����ͨ��x mol CO, t ��ʱ��Ӧ�ﵽƽ�⡣��ʱFeO(s)ת����Ϊ50%����x��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪þ��ϡ���ᷴӦʱ��ÿ��1 molHNO3 ��Ӧ������0.8mol����ת�ƣ���ʱ����Ļ�ԭ��������� �� ��
A��NO2 B��N2O C��N2O3 D��NO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������Һ�У����ʵ����ʵ���Ũ��Ϊ1 mol•L��1����( )
A.��40 g NaOH����1 Lˮ�����õ���Һ
B.��22.4 L HCl����ˮ���1 L��Һ
C.��K�������ʵ���Ϊ2 mol��K2SO4��Һ1 L
D.��0.5 mol•L��1��NaNO3��Һ100 mL����������50 gˮ����Һ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com