
��ӦaA(g)+bB(g)  cC(g)(��H��0)�ڵ��������½��С��ı�������Ӧ�������ڢ����ϵ�и�����Ũ����ʱ��仯����������ͼ��ʾ���ش����⣺
cC(g)(��H��0)�ڵ��������½��С��ı�������Ӧ�������ڢ����ϵ�и�����Ũ����ʱ��仯����������ͼ��ʾ���ش����⣺
(1)��Ӧ�Ļ�ѧ����ʽ�У�a: b : cΪ_____________
(2)A��ƽ����Ӧ����vI(A)��v��(A)��v��(A)�Ӵ�С���д���Ϊ_________��
(3)B��ƽ��ת���ʦ�I(B)������(B)��
���� (B) �� ������ _____ ��
��ֵ��__________��
(4) �ɵ�һ��ƽ��ڶ���ƽ�⣬ƽ�� �ƶ��ķ�����______________����ȡ�Ĵ�ʩ��____________��

(5) �Ƚϵڢ�η�Ӧ�¶�(T2)�͵ڢ�η�Ӧ�¶�(T3)�ĸߵͣ�T2 T3
(��>�� >��=)���жϵ�������________________________________________

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з�Ӧ�У��������뻹ԭ�����ʵ���֮��Ϊ1��2����
A��3S��6NaOH===2Na2S��Na2SO3��3H2O
B��2CH3COOH��Ca(ClO)2===2HClO��Ca(CH3COO)2
C��I2��2NaClO3===2NaIO3��Cl2
D��4HCl(Ũ)��MnO2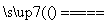 MnCl2��Cl2����2H2O
MnCl2��Cl2����2H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���г����µ�������Һ��
��0.01 mol/L CH3COOH��Һ��
��0.01 mol/L HCl��Һ��
��pH��12�İ�ˮ��
��pH��12��NaOH��Һ��
��0.01 mol/L CH3COOH��Һ��pH��12�İ�ˮ�������Ϻ�������Һ��
��0.01 mol/L HCl��Һ��pH��12��NaOH��Һ��������������Һ��
(1)����ˮ�ĵ���̶�������________(����ţ���ͬ)��ˮ�ĵ���̶���ͬ����____________��
(2)�����ڡ��ۻ�Ϻ�������ҺpH��7����������Һ���������________��(�>������<������)��
(3)��������Һͬ��ϡ��10������Һ��pH��
��________�ڣ���________�ܣ���________��(�>������<������)��
(4)���١��ܻ�ϣ�����c(CH3COO��)>c(H��)��������Һ���ܳ�________(�����)��
A�����ԡ���B�����ԡ���C������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ܱ������н������·�X2(g)+Y2(g) 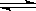 2Z(g)����֪X2��Y2��Z����ʼŨ�ȷֱ�Ϊ0.1mol/L��0.3mol/L��0.2mol/L����һ�������£�����Ӧ�ﵽƽ��ʱ�������ʵ�Ũ���п����� ( )
2Z(g)����֪X2��Y2��Z����ʼŨ�ȷֱ�Ϊ0.1mol/L��0.3mol/L��0.2mol/L����һ�������£�����Ӧ�ﵽƽ��ʱ�������ʵ�Ũ���п����� ( )
A�� ZΪ0.3mol/L B�� Y2Ϊ0.4mol/L C��X2Ϊ0.2mol/L D. ZΪ0.4mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
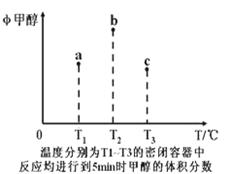
��֪CO��H2��һ�������ºϳɼ״��ķ�ӦΪ��CO(g)+2H2(g) CH3OH(g)�������ݻ���Ϊ1L��a��b��c�����ܱ������зֱ����1mol CO��2mol H2�Ļ�����壬�����ڲ�ͬ�¶��½��з�Ӧ��5minʱ���������ݵĹ�ϵ��ͼ��ʾ������˵����ȷ����
CH3OH(g)�������ݻ���Ϊ1L��a��b��c�����ܱ������зֱ����1mol CO��2mol H2�Ļ�����壬�����ڲ�ͬ�¶��½��з�Ӧ��5minʱ���������ݵĹ�ϵ��ͼ��ʾ������˵����ȷ����
| A���÷�Ӧ������Ӧ�ġ�H>0 |
| B����Ӧ���е�5minʱ��a������v��=v�� |
| C����ѹ�ɽ�b�е�ƽ��״̬ת���c�е�ƽ��״̬ |
| D���ﵽƽ��ʱ��a��b��c��CO��ת����Ϊb>a>c |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з�ɢϵ���ܷ��������������ǣ� ����
A������������ �� B��ţ�� C��������Һ���� D���̡��ơ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����з�Ӧ�У� HCl ������������ �� ��
A��NaOH+HCl��NaCl+H2O
B��Zn+2HCl=ZnCl2+H2��
C��MnO2+4HCl(Ũ)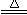 MnCl2+2H2O+Cl2��
MnCl2+2H2O+Cl2��
D��CuO+2HCl=CuCl2+H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Һ�����е���Ҫ�ɷ�֮һ�Ƕ��飬��10 kg������ȫȼ�ղ����ɶ�����̼��Һ̬ˮʱ���ų�����Ϊ5��105 kJ����д������ȼ�շ�Ӧ���Ȼ�ѧ����ʽ__________________________��
��֪1 molҺ̬ˮ����ʱ��Ҫ����44 kJ����������1 mol������ȫȼ�ղ�������̬ˮʱ�ų�������Ϊ_______________kJ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�йآ�100 mL 0.1 mol/L NaHCO3����100 mL 0.1 mol/L Na2CO3������Һ����������ȷ����(����)
A����Һ��ˮ�������H����������>��
B����Һ�������ӵ����ʵ���Ũ��֮�ͣ���>��
C������Һ�У�c(CO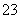 )>c(H2CO3)
)>c(H2CO3)
D������Һ�У�c(HCO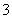 )>c(H2CO3)
)>c(H2CO3)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com