
����15�֣� I��9�֣�д�������Ȼ�ѧ��Ӧ����ʽ
��1��N2 (g)��H2(g)��Ӧ����1molNH3(g)���ų�46.1KJ������
��2��1molC2H5OH(l)��ȫȼ������CO2(g)��H2O(l)���ų�1366.8KJ������
��3��1molC��ʯī��������H2O(g)��Ӧ����131.3KJ����
II .��6�֣���1����ѧ��Ӧ�о������������ı仯����ѧ���Ķ��Ѻ��γ��Ƿ��������仯����Ҫԭ���������л�ѧ���γ�ʱ��__________��������ų��������ա��������һ����ѧ��Ӧ����ѧ������ʱ�������仯���ڻ�ѧ���γ�ʱ�������仯����÷�Ӧ����_________��Ӧ�����һ����ѧ��Ӧ����Ӧ����������������������������ͼ��ʾ�Ĺ�ϵ����÷�Ӧ����__________��Ӧ��

��2����ͬ��ʽ�����������ת�����磺��ѧ�ܺ͵��ܡ�����֮����ת������ͼ��һ��ԭ��ع���ԭ����ʾ��ͼ���Իش�

�ٴ������Ƕȿ���������____________��ת��Ϊ____________�ܣ�
��װ����ZnΪ____________����[��Դ:ѧ,��,��]
 ȫ��������ϵ�д�
ȫ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����15�֣� I��9�֣�д�������Ȼ�ѧ��Ӧ����ʽ
��1��N2 (g)��H2(g)��Ӧ����1molNH3(g)���ų�46.1KJ������
��2��1molC2H5OH(l)��ȫȼ������CO2(g)��H2O(l)���ų�1366.8KJ������
��3��1molC��ʯī��������H2O(g)��Ӧ����131.3KJ����
II .��6�֣���1����ѧ��Ӧ�о������������ı仯����ѧ���Ķ��Ѻ��γ��Ƿ��������仯����Ҫԭ���������л�ѧ���γ�ʱ��__________��������ų��������ա��������һ����ѧ��Ӧ����ѧ������ʱ�������仯���ڻ�ѧ���γ�ʱ�������仯����÷�Ӧ����_________��Ӧ�����һ����ѧ��Ӧ����Ӧ����������������������������ͼ��ʾ�Ĺ�ϵ����÷�Ӧ����__________��Ӧ��
��2����ͬ��ʽ�����������ת�����磺��ѧ�ܺ͵��ܡ�����֮����ת������ͼ��һ��ԭ��ع���ԭ����ʾ��ͼ���Իش�
�ٴ������Ƕȿ���������____________��ת��Ϊ____________�ܣ�
��װ����ZnΪ____________����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����15�֣�I��8�֣� (1)��4�֣�Ϊ�Ƚ�Fe3+ ��Cu2+ ��H2O2�ֽ�Ĵ�Ч�����ס�����λͬѧ�ֱ��������ͼ��ʾ��ʵ�顣
�ס�����ͬѧ��ͨ���۲� �����ԱȽϵó����ۡ��۲���ͬѧ�ķ���������Ϊ ����ס����ҡ����������� ������ ��
(2) ��4�֣���ͬѧ�������������ʵ����֤��������ø��H2O2�ֽ�����ã����ʼ���Ƭ�к��й�������ø����
�����Թ�A�м���3%��˫��ˮ5 mL���ټ������ʵļ���Ƭ1 g�������������
�����Թ�B�м���3%��˫��ˮ5 mL���ټ�������ļ���Ƭ1 g�������������
�����Թ�C�м���3%��˫��ˮ3 mL��10%��H2SO42mL���ټ������ʵļ���Ƭ1 g�������������
������ʵ��õ��Ľ�����
_______________________________________________________________________��
_______________________________________________________________________��
д�����в�������Ļ�ѧ����ʽ��_____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ�������Ƕ��и߶���ѧ������������⻯ѧ�Ծ� ���ͣ������
����15�֣� I��9�֣�д�������Ȼ�ѧ��Ӧ����ʽ
��1��N2 (g)��H2(g)��Ӧ����1molNH3(g)���ų�46.1KJ������
��2��1molC2H5OH(l)��ȫȼ������CO2(g)��H2O(l)���ų�1366.8KJ������
��3��1molC��ʯī��������H2O(g)��Ӧ����131.3KJ����
II .��6�֣���1����ѧ��Ӧ�о������������ı仯����ѧ���Ķ��Ѻ��γ��Ƿ��������仯����Ҫԭ���������л�ѧ���γ�ʱ��__________��������ų��������ա��������һ����ѧ��Ӧ����ѧ������ʱ�������仯���ڻ�ѧ���γ�ʱ�������仯����÷�Ӧ����_________��Ӧ�����һ����ѧ��Ӧ����Ӧ����������������������������ͼ��ʾ�Ĺ�ϵ����÷�Ӧ����__________��Ӧ��
��2����ͬ��ʽ�����������ת�����磺��ѧ�ܺ͵��ܡ�����֮����ת������ͼ��һ��ԭ��ع���ԭ����ʾ��ͼ���Իش�
�ٴ������Ƕȿ���������____________��ת��Ϊ____________�ܣ�
��װ����ZnΪ____________����[��Դ:ѧ,��,��]
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��09��10�껴���л�������һ��ѧ����ĩ������Ի�ѧ�� ���ͣ�ʵ����
����15�֣�I��8�֣�(1)��4�֣�Ϊ�Ƚ�Fe3+ ��Cu2+ ��H2O2�ֽ�Ĵ�Ч�����ס�����λͬѧ�ֱ��������ͼ��ʾ��ʵ�顣
�ס�����ͬѧ��ͨ���۲� �����ԱȽϵó����ۡ��۲���ͬѧ�ķ���������Ϊ ����ס����ҡ����������������� ��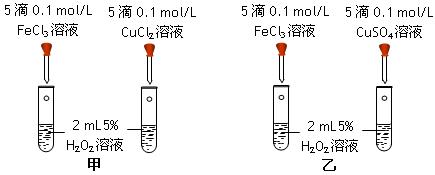
(2) ��4�֣���ͬѧ�������������ʵ����֤��������ø��H2O2�ֽ�����ã����ʼ���Ƭ�к��й�������ø����
�����Թ�A�м���3%��˫��ˮ5 mL���ټ������ʵļ���Ƭ1 g�������������
�����Թ�B�м���3%��˫��ˮ5 mL���ټ�������ļ���Ƭ1 g�������������
�����Թ�C�м���3%��˫��ˮ3 mL��10%��H2SO42 mL���ټ������ʵļ���Ƭ1 g�������������
������ʵ��õ��Ľ�����
_______________________________________________________________________��
_______________________________________________________________________��
д�����в�������Ļ�ѧ����ʽ��_____________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com