��ҵ�ϳɰ����Ʊ�����һ���������������������
��1���ٹ�ҵ����ʱ����ȡ������һ����ӦΪ��CO+H
2O��g��?CO
2+H
2��850��ʱ����1L�ܱ������г���0.3mol CO��0.2molH
2O��g������Ӧ4min����ƽ�⣬��ϵ��c��H
2��=0.12mol?L
-1��CO��ƽ��Ũ��Ϊ
0.18mol/L
0.18mol/L
ת����Ϊ
40%
40%
���¶��´˷�Ӧ��ƽ�ⳣ��K=
1
1
�������������
����850��ʱ���Ա��е����ʵ���Ͷ����ݷ�Ӧ���У��������淴Ӧ������е���
A
A
��ѡ��A��B��C��D��E��
|
A |
B |
C |
D |
E |
| n��CO2�� |
3 |
l |
0 |
1 |
l |
| n��H2�� |
2 |
l |
0 |
1 |
2 |
| n��CO�� |
1 |
2 |
3 |
0.5 |
3 |
| n��H2O�� |
5 |
2 |
3 |
2 |
l |
��2���ϳ����з�����ӦN
2��g��+3H
2��g��?2NH
3��g����H��0���±�Ϊ��ͬ�¶��¸÷�Ӧ��ƽ�ⳣ�����ɴ˿���֪������T
1��
��
300�棨���������������=������
| T/��C |
T1 |
300 |
T2 |
| K |
1.00��107 |
2.45��105 |
1.88��103 |
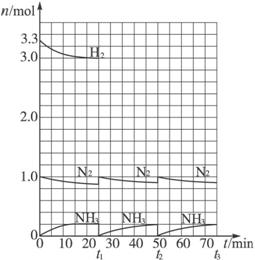
��3��N
2��H
2���������������´�145��Ϳ�ʼ��Ӧ����ͬ�¶���NH
3����ͼ1��ʾ���¶ȸ���900��ʱ��NH
3�����½���ԭ����
900��ʱ��Ӧ�ﵽƽ��״̬���������¶�ƽ�������ƶ�
900��ʱ��Ӧ�ﵽƽ��״̬���������¶�ƽ�������ƶ�
��
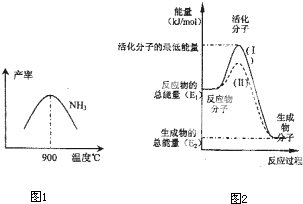
��4���ڻ�ѧ��Ӧ��ֻ�м�����������ƽ�������ߵö�ķ�Ӧ����ӷ�����ײʱ�ſ��ܷ�����ѧ��Ӧ����Щ���ӱ���Ϊ����ӣ�ʹ��ͨ���ӱ�ɻ���������ṩ������ȵ������л�ܣ��䵥λͨ����kJ?mol
-1��ʾ��������۲�ͼ2���ش����⣮
ͼ����ʾ��Ӧ��
����
����
������ȡ����ȡ�����Ӧ���÷�Ӧ�ġ�H=
-��E1-E2��kJ/mol
-��E1-E2��kJ/mol
���ú�E
1��E
2E�Ĵ���ʽ��ʾ������֪�Ȼ�ѧ����ʽ��H
2��g��+
O
2��g��=H
2O��g����H=-241.8kJ?mol
-1���÷�Ӧ�Ļ��Ϊ167.2kJ?mol
-1�������淴Ӧ�Ļ��Ϊ
409kJ/mol
409kJ/mol
��
��5�����᳧��β��ֱ���ŷŽ���Ⱦ������Ŀǰ��ѧ��̽������ȼ�������еļ���Ƚ����������ﻹԭΪ������ˮ����Ӧ����Ϊ��
CH
4��g��+4NO
2��g��=4NO��g��+CO
2��g��+2H
2O��g����H=-574kJ?mol
-1CH
4��g��+4NO��g��=2N
2��g��+CO
2��g��+2H
2O��g����H=-1160kJ?mol
-1�����ֱ�ӽ�NO
2��ԭΪN
2���Ȼ�ѧ����ʽΪ��
CH4��g��+2NO2��g��=CO2��g��+2H2O��g��+N2��g����H=-867kJ?mol-1
CH4��g��+2NO2��g��=CO2��g��+2H2O��g��+N2��g����H=-867kJ?mol-1
��

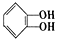 ���ݶ��ǻ�����ȩ����CH3CH2OH�У����γɷ��Ӽ��������____�����γɷ������������____�������γ��������____��
���ݶ��ǻ�����ȩ����CH3CH2OH�У����γɷ��Ӽ��������____�����γɷ������������____�������γ��������____��  ���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
 ������һ�������Դ������ͨ�����ַ����Ƶã�
������һ�������Դ������ͨ�����ַ����Ƶã�

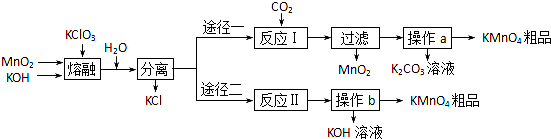
 H2��g��+CO2��g����ƽ�ⳣ�����¶ȵı仯�����
H2��g��+CO2��g����ƽ�ⳣ�����¶ȵı仯����� 2CO��g��ƽ�ⳣ��K��
2CO��g��ƽ�ⳣ��K�� CO��g��+H2��g�� ƽ�ⳣ��K1��
CO��g��+H2��g�� ƽ�ⳣ��K1�� H2��g��+CO2��g�� ƽ�ⳣ��K2��
H2��g��+CO2��g�� ƽ�ⳣ��K2��