
|
��ҵ��Ŀǰ���ü�����Fe��Co��Ni�ȹ��ɽ������£������ѽ�����������CH4(g)
| |
| [����] | |
A�� |
������ϵѹǿ����������ת���� |
B�� |
ͬһ�¶��£����ŷ�Ӧ�Ľ��У����ڲ���̿�ڵĶѻ�����ʹ�������ʹ����� |
C�� |
�����ѽ����ڷ��ȷ�Ӧ |
D�� |
��1500������ʱ�������ת���ʺܸߣ��������ò���̿�ڣ�����Ϊ�÷�Ӧ�����ȷ�Ӧ |
 �ܿ�����ĩ��̾�ϵ�д�
�ܿ�����ĩ��̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ���� |
| ||
| ���� |
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �� �� | ȼ���ȣ�kJ?mol-1�� |
| H2��g�� | -285.8 |
| CO��g�� | -283.0 |
| CH4��g�� | -890.3 |
| ��Ӧ�¶�/�� | ƽ�ⳣ�� | ��Ӧ�¶�/�� | ƽ�ⳣ�� |
| 0 | 667.30 | 200 | 1.909��10-2 |
| 100 | 12.92 | 300 | 2.42��10-4 |
 HOCH2CH2CHO
HOCH2CH2CHO  HOCH2CH2CH2OH
HOCH2CH2CH2OH
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
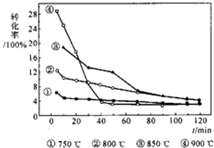 ?C��s��+2H2��g������ͼ���¶ȶԼ���ת���ʵ�Ӱ�죬�����й�˵����ȷ���ǣ�������
?C��s��+2H2��g������ͼ���¶ȶԼ���ת���ʵ�Ӱ�죬�����й�˵����ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���ս̰���л�ѧѡ��1 1.3���������ķ��ദ����ϰ���������棩 ���ͣ������
(1)�������ö�����̼��������������������ٽ���ҵ��չ��
���ڳ�ѹ�£���78 ��ʱ��������̼��������ɹ�̬������̼���׳Ƹɱ���ijѧ�����һ�б�����±��������кܶ���Ϊ���������ɱ������й��ڸɱ���������ȷ������________��
A�����ɱ��������
B���øɱ���ͨ�紦��Ȼ����
C��ֱ�ӽ��ɱ�������ˮ��
D������ȡ�ɱ����������
���ڲ�ú��ҵ�ϣ��Ѹɱ���ըҩ����һ�𣬼�����ǿ��ը���������ܷ�ֹ���֡�����ǿ��ը������ԭ����________________________________���ܷ�ֹ���ֵ�ԭ����______________________________________________________________________
________________________________________________________________________��
��ʹ�ô�ͳ������ʹ������̼��ijЩ�л��ﷴӦ�����������ϣ�����Ӧʱ�䳤��Ŀǰ�������������������˴����ı�����������____________________________��ʹ�����������ܼ��ٰ�ɫ��Ⱦ����Ϊ�������������£�����ʵ��100%��________��
(2)Ŀǰ�����ڶ�����̼�Ƿ�Ϊ������Ⱦ���в�ͬ�Ĺ۵㡣��Ϊ��������̼���Ǵ�����Ⱦ���������________��
�ٶ�����̼����Ҫ�Ļ���ԭ�ϡ��ڶ�����̼��ֲ�������õı���ԭ�ϡ��۶�����̼����ɫ����ζ����������
�ܳ�������̼�⣬���顢һ��������Ҳ����������
A���٢� B���ڢ�
C���ۢ� D���٢�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com