
�Ҵ�����Ҫ���л�����ԭ�ϣ�������ϩ����ֱ��ˮ�Ϸ���������ˮ�Ϸ��������ش��������⣺
(1)���ˮ�Ϸ���ָ�Ƚ���ϩ��Ũ���ᷴӦ��������������(C2H5OSO3H)����ˮ�������Ҵ���д����Ӧ��Ӧ�Ļ�ѧ����ʽ��________________________________________��
(2)��֪��
�״�����ˮ��Ӧ
2CH3OH(g)===CH3OCH3(g)��H2O(g)
��H1����23.9 kJ��mol��1
�״���ϩ���ķ�Ӧ
2CH3OH(g)===C2H4(g)��2H2O(g)
��H2����29.1 kJ��mol��1
�Ҵ����칹����Ӧ��C2H5OH(g)===CH3OCH3(g)
��H3����50.7 kJ��mol��1
����ϩ����ֱ��ˮ�Ϸ�ӦC2H4(g)��H2O(g)===C2H5OH(g)�Ħ�H________kJ��mol��1������ˮ�Ϸ���ȣ�����ֱ��ˮ�Ϸ����ŵ���____________________________________��
(3)��ͼ��ʾΪ����ֱ��ˮ�Ϸ�����ϩ��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ[����n(H2O)��n(C2H4)��1��1]��
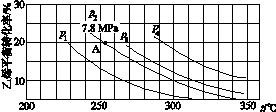
����ʽ������ϩˮ�����Ҵ���Ӧ��ͼ��A���ƽ�ⳣ��Kp��____________________(��ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ����ѹ�����ʵ�������)��
��ͼ��ѹǿ(p1��p2��p3��p4)�Ĵ�С˳��Ϊ____��������___________________��
������ֱ��ˮ�Ϸ������õĹ�������Ϊ������/������Ϊ��������Ӧ�¶�290 �桢ѹǿ6.9 MPa��n(H2O)��n(C2H4)��0.6��1����ϩ��ת����Ϊ5%����Ҫ��һ�������ϩת���ʣ����˿����ʵ��ı䷴Ӧ�¶Ⱥ�ѹǿ�⣬���ɲ�ȡ�Ĵ�ʩ��________________________________________________________________________��
________________________________________________________________________��
(1)C2H4��H2SO4�D��C2H5OSO3H��C2H5OSO3H��H2O�D��C2H5OH��H2SO4��(2)��45.5����ȾС����ʴ��С�ȡ�(3)��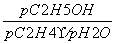 ��
��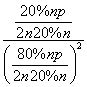 ��
��
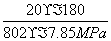 ��0.07(MPa)��1����p1<p2<p3<p4����Ӧ���������٣���ͬ�¶��£�ѹǿ������ϩת�������
��0.07(MPa)��1����p1<p2<p3<p4����Ӧ���������٣���ͬ�¶��£�ѹǿ������ϩת�������
�۽������Ҵ�Һ����ȥ������n(H2O)��n(C2H4)��
[����] (1)����������Ϣ��д������ϩ��Ũ������ˮ�Ϸ����Ҵ��ķ�ӦΪC2H4��H2SO4�D��C2H5OSO3H ��C2H5OSO3H��H2O�D��C2H5OH��H2SO4��(2)���ݸ�˹���ɢ٣��ڣ��۵ã�C2H4(g)��H2O(g)===C2H5OH(g)����H����45.5 kJ��mol��1�����ˮ�Ϸ����õ�Ũ�����ǿ��ʴ�����ʣ��������ֱ��ˮ�Ϸ�������ȾС����ʴ��С���ŵ㡣(3)������ʼʱC2H4��H2O(g)�����ʵ�����Ϊn������C2H4��ת����Ϊ20%����ƽ��ʱC2H4��H2O(g)��C2H5OH�����ʵ����ֱ�Ϊ80%n��80%n��20%n����Kp��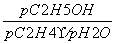 ��
��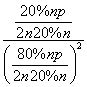 ��
��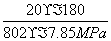 ��0.07(MPa)��1��������ѹǿ��ƽ�⽫�����ƶ��������C2H4��ת���ʣ���ѹǿp1��p2��p3��p4����Ϊ��ʹƽ�������ƶ��������Խ��Ҵ�Һ����ʱ���룬������n (H2O)��n (C2H4) ֮�ȵȴ�ʩ��
��0.07(MPa)��1��������ѹǿ��ƽ�⽫�����ƶ��������C2H4��ת���ʣ���ѹǿp1��p2��p3��p4����Ϊ��ʹƽ�������ƶ��������Խ��Ҵ�Һ����ʱ���룬������n (H2O)��n (C2H4) ֮�ȵȴ�ʩ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и���ԭ��������ʾ������Ԫ���У��ܹ��γɹ��ۻ��������
A�� 11��17 B��11��10 C��1��17 D��1��8
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���¹��ڻ�ѧʵ ���С������˵������ȷ����(����)
���С������˵������ȷ����(����)
�ټ����Թ�ʱ���Ⱦ��ȼ��ȣ���ֲ����ȣ�
������ˮ���ռ�����ʱ�����Ƴ����ܺƾ��ƣ�
����ȡ����ʱ���ȼ��װ�������Ժ�װҩƷ��
��ʹ������ƿ����Һ©�����ζ���ǰ�� �ȼ���Ƿ�©ˮ��ϴ�Ӹɾ���
�ȼ���Ƿ�©ˮ��ϴ�Ӹɾ���
����H2��ԭCuOʵ��ʱ���ȼ���CuO��ͨH2����Ӧ��Ϻ��ȳ��ƾ��ƴ��Թ���ȴ��ֹͣͨH2��
A���������⡡������ B���������� C���������� D��ȫ����ȷ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
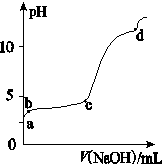 (��) �����£���30ml�� Al2(SO4)3��Һ�У���μ���1.0 mol��L��1 NaOH��Һ��ʵ������ҺpH��NaOH��Һ����ı仯������ͼ��ʾ��
(��) �����£���30ml�� Al2(SO4)3��Һ�У���μ���1.0 mol��L��1 NaOH��Һ��ʵ������ҺpH��NaOH��Һ����ı仯������ͼ��ʾ��
��1��д��a����Һ�����Ե����ӷ���ʽ��
��2����c��ʱV��NaOH��Ϊ90ml����Al2(SO4)3��Һ�����ʵ���Ũ��ԼΪ��
��3��д��b��c�η�Ӧ�����ӷ���ʽ�� ��
(4) d��ʱ��V��NaOH��ԼΪ 

(��)��������Ũ�Ⱦ�Ϊ0.5 mol/L��������Һ��
��Na2CO3��Һ����NaHCO3��Һ����HCl��Һ���ܰ�ˮ
(1) ����м��������Ȼ�粒��壬��ʱc(NH4+)/c(OH��)��ֵ________(���������С�����䡱)��
(2)�����ۺܵ͢���Һ��Ϻ���Һǡ�ó��������� ǰ�۵����________�ܵ����(����ڡ�����С�ڡ����ڡ�)����ʱ��Һ������Ũ���ɴ�С��˳����________________��
ǰ�۵����________�ܵ����(����ڡ�����С�ڡ����ڡ�)����ʱ��Һ������Ũ���ɴ�С��˳����________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ݻ�Ϊ1.00 L�������У�ͨ��һ������N2O4��������ӦN2O4(g)2NO2(g)�����¶����ߣ�����������ɫ���
�ش��������⣺
(1)��Ӧ�Ħ�H________0(����ڡ���С�ڡ�)��100 ��ʱ����ϵ�и�����Ũ����ʱ��仯��ͼ��ʾ����0��60 sʱ�Σ���Ӧ����v(N2O4)Ϊ________mol��L��1��s��1����Ӧ��ƽ�ⳣ��K1Ϊ________��
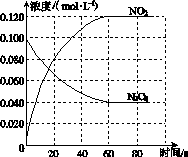
(2)100 ��ʱ��ƽ��ı䷴Ӧ�¶�ΪT��c(N2O4)��0.002 0 mol��L��1��s��1��ƽ�����ʽ��ͣ���10 s�ִﵽƽ�⡣
��T________100 ��(����ڡ���С�ڡ�)���ж�������____________________________��
����ʽ�����¶�Tʱ��Ӧ��ƽ�ⳣ��K2��_______________________________________
________________________________________________________________________��
(3)�¶�Tʱ��Ӧ��ƽ�����Ӧ�������ݻ�����һ�룬ƽ����________(�����Ӧ�����淴Ӧ��)�����ƶ����ж�������__________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£���1 mol��CuSO4��5H2O(s)����ˮ��ʹ��Һ�¶Ƚ��ͣ���ЧӦΪ��H1����1 mol��CuSO4(s)����ˮ��ʹ��Һ�¶����ߣ���ЧӦΪ��H2��CuSO4��5H2O���ȷֽ�Ļ�ѧ����ʽΪCuSO4��5H2O(s) CuSO4(s)��5H2O(l)����ЧӦΪ��H3���������ж���ȷ����(����)
CuSO4(s)��5H2O(l)����ЧӦΪ��H3���������ж���ȷ����(����)
A����H2����H3
B����H1����H3
C����H1����H3����H2
D����H1����H2����H3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��̵�ص����С�������������dz��õ�һ�ε�ء��õ�ط�Ӧԭ����ͼ��ʾ�����е����LiClO4�����ڻ���л��ܼ��У�Li��ͨ�������Ǩ����MnO2�����У�����LiMnO2�� �ش��������⣺

(1)���·�ĵ�����������________������________����(����ĸ)
(2)���������ӦʽΪ__________________________��
(3)�Ƿ����ˮ�������еĻ���л��ܼ���________(��ǡ���)��ԭ����________________________________________________��
(4)MnO2����KOH��KClO3�ڸ����·�Ӧ������K2MnO4����Ӧ�Ļ�ѧ����ʽΪ____________________________________��K2MnO4��������Һ���绯������KMnO2��MnO2�����ʵ���֮��Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ�������(����)
A����Ba(OH)2��Һ�еμ�ϡ���
Ba2����2OH����2H����SO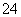 ===BaSO4����2H2O
===BaSO4����2H2O
B�����Խ�����KMnO4����H2O2��
2MnO ��5H2O2��6H��===2Mn2����5O2����8H2O
��5H2O2��6H��===2Mn2����5O2����8H2O
C�������ʵ�����MgCl2��Ba(OH)2��HCl��Һ��ϣ�Mg2����2OH��===Mg(OH)2��
D��Ǧ�����س��ʱ��������Ӧ��PbSO4��2H2O��2e��===PbO2��4H����SO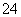
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и�������ʾ��ͼһ�µ���
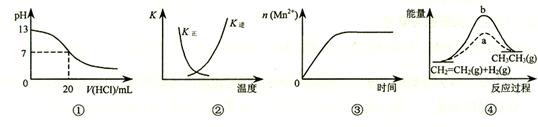
A��ͼ�ٱ�ʾ25��ʱ����0.1 mol��L��1����ζ�20 mL 0.1 mol��L��1 NaOH��Һ����Һ��pH�����������ı仯
B��ͼ�������߱�ʾ��Ӧ2SO2(g) + O2(g)  2SO3(g)����H < 0 �����淴Ӧ��ƽ�ⳣ��K���¶ȵı仯
2SO3(g)����H < 0 �����淴Ӧ��ƽ�ⳣ��K���¶ȵı仯
C��ͼ�۱�ʾ10 mL 0.01 mol��L��1 KMnO4 ������Һ�������0.1 mol��L��1 H2C2O4��Һ���ʱ��n(Mn2+) ��ʱ��ı仯
D��ͼ����a��b���߷ֱ��ʾ��ӦCH2��CH2 (g) + H2(g) CH3CH3(g)����H< 0ʹ�ú�δʹ�ô���ʱ����Ӧ�����е������仯
CH3CH3(g)����H< 0ʹ�ú�δʹ�ô���ʱ����Ӧ�����е������仯
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com