
��NH4HCO3��NH4Cl��Na2CO3��xH2O�Ļ���ﹲ11.62g������44mL5mol��L-1������NaOH��Һ���ȳ�ַ�Ӧ��ʹ�ų�������ͨ����ʯ�ң��ռ�����״���µ�����3.36L��������Һ�м���30mL2.5mol��L-1H2SO4��Һ�����Լ���ʹ������ȫ�ų����ռ�����״���¸��������1.344L���ѷ�Ӧ�����Һϡ�͵�100mL�����H+��Ũ��Ϊ0.1mol��L-1����
��1��ԭ������и����ʵ�����
��2��Na2CO3��xH2O�е�xֵ��
����ʾ����ο�����NaOH��Һ��Ӧ���ɰ�����
�⣺������H+��0.1mol/L´0.1L=0.01mol H2SO4��Na2CO3��Ӧ����CO2Ϊ1.344L��0.06mol����ʱ����H2SO40.06mol�к�NaOH�����ĵ�H2SO4Ϊ�� 0.03L´2.5mol��L- �μӷ�Ӧ��NaOH�� 0.044L´5mol��L-1-2´0.01mol=0.2mol ����NH33.36L����0.15mol ������NaOH0.15mol�к�HCO3-��NaOHΪ0.05mol ��NH4HCO3Ϊ0.05mol����3.95g��NH4ClΪ0.1mol����5.35g��Na2CO3��xH2OΪ2.32g ��n(CO2)=0.06mol n(NH4HCO3)=0.05mol ��Na2CO3��cH2OΪ0.01mol 0.01mol´(106+18x)g��mol-1=2.32g ��c=7 ����
|


 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| 50m |
| 13a |
| 50m |
| 13a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ����������һ�и�����ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ�ʵ����
��16�֣�ʵ������NH4HCO3��NaHSO3�ĸ����״����ij��ȤС��Ϊ�ⶨ����NH4HCO3�ĺ�������������ڻ�����м����ʵ�鷽�����ⶨ�������������ش��������⣺
�Ż�ѧС����Ҫѡ��������ҩƷ����������ʵ�顣��ͼ��ÿ������װ��ֻѡ����һ�Σ�����̨�ȹ̶�����δ������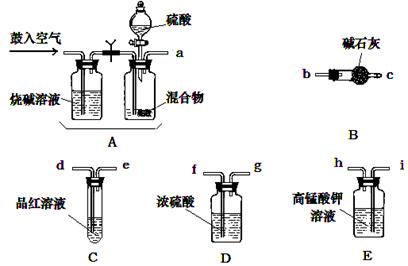
�밴�����������ҵķ�����������˳���ǣ��������Ľӿ���ĸa��b��������
a�� �� �� �� �� f ��g �� �� ��
��ʵ�鿪ʼ����������ǰ��Aװ����Ҫ��������������� �������Aװ���ٴι�������������� ��
�� Eװ�õ������� ��
��ʵ���У���Cƿ����Һ��ɫ����ⶨ������ܻ�ƫ ����ߡ��͡�����Ϊ��ȷ
��ʵ����Cƿ��Һ����ɫ����ȡ��Ʒ������Ϊm g��ʵ��ǰ E����װ a mol��L��1��KMnO4
��Һ�����V�������� mL��
�����������Ʒ����Ϊ13.1g��������ú��ʯ������4.4g����������NH4HCO3����
������Ϊ ��
�ʴӶ����ⶨ��ȷ�Կ��ǣ�����װ�û�Ӧ����һ���ĸĽ��� ��
�����õ������ԭ��������H��1 C��12 N��14 O��16 Na��23 S��32
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ�����и�����ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��16�֣�ʵ������NH4HCO3��NaHSO3�ĸ����״����ij��ȤС��Ϊ�ⶨ����NH4HCO3�ĺ�������������ڻ�����м����ʵ�鷽�����ⶨ�������������ش��������⣺
�� ��ѧС����Ҫѡ��������ҩƷ����������ʵ�顣��ͼ��ÿ������װ��ֻѡ����һ�Σ�����̨�ȹ̶�����δ������

�밴�����������ҵķ�����������˳���ǣ��������Ľӿ���ĸa��b��������
a�� �� �� �� �� f ��g �� �� ��
�� ʵ�鿪ʼ����������ǰ��Aװ����Ҫ��������������� �������Aװ���ٴι�������������� ��
�� Eװ�õ������� ��
�� ʵ���У���Cƿ����Һ��ɫ����ⶨ������ܻ�ƫ ����ߡ��͡�����Ϊ��ȷ
��ʵ����Cƿ��Һ����ɫ����ȡ��Ʒ������Ϊm g��ʵ��ǰ E����װ a mol��L��1��KMnO4
��Һ�����V�������� mL��
�� ���������Ʒ����Ϊ13.1 g��������ú��ʯ������4.4 g����������NH4HCO3����
������Ϊ ��
�� �Ӷ����ⶨ��ȷ�Կ��ǣ�����װ�û�Ӧ����һ���ĸĽ��� ��
�����õ������ԭ��������H��1 C��12 N��14 O��16 Na��23 S��32
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ�ϲ��а�һ��ѧ���鶼��ѧ��������ѧ�������ϣ����л�ѧ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com