
��ϩ�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ������ϩΪ��Ҫԭ�Ϻϳ���Ҫ���л�������·������ͼ��ʾ����ش��������⡣

��1����ϩ�Ľṹ��ʽ___________������ʽ_______________��
��2��A�������������ŵ�������__________________��
��3����Ӧ�ڵķ�Ӧ������_______________________��
��4����Ӧ�۵Ļ�ѧ����ʽ��___________________________________��
��5������ϩΪԭ�Ͽɺϳ��л��߷��ӻ�����D����Ӧ�ٵĻ�ѧ����ʽ��
____________________________________________________________��
��6�����������У�����ͨ����ϩ�ӳɷ�Ӧ�õ�����_____������ţ���
a. CH3CH3 b. CH3CHCl2 c. CH3CH2Br
��10�֣���1��CH2=CH2��1�֣� 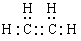 ��1�֣�
��1�֣�
��2���ǻ���1�֣���3���ӳɷ�Ӧ��1�֣�
��4��2CH3CH2OH+O2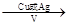 2CH3CHO+
2H2O��2�֣���д��������������1�֣�
2CH3CHO+
2H2O��2�֣���д��������������1�֣�
��5��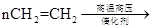
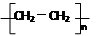 ��2�֣�д��һ�����������֣���д������1�֣�
��2�֣�д��һ�����������֣���д������1�֣�
��6��ac��2�֣���ѡΪ0�֣���ѡΪ1�֣�
��������
�����������ϩ����̼̼˫�����ܷ����Ӿ۷�Ӧ�����ɾ���ϩ������D�Ǿ���ϩ����ϩҲ�ܺ�ˮ�����ӳɷ�Ӧ�����Ҵ�����A���Ҵ����Ҵ�����������Ӧ����B������B����ȩ����ȩ�����������ᡣ
��1����ϩ����̼̼˫�����ṹ��ʽ��CH2��CH2������ʽ��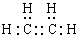 ��
��
��2��A���Ҵ������еĹ��������ǻ���
��3����Ӧ�����Ҵ���ˮ�����ӳɷ�Ӧ�����Ҵ����л���Ӧ�����Ǽӳɷ�Ӧ��
��4����Ӧ�����Ҵ��Ĵ�����������ȩ����Ӧ�Ļ�ѧ����ʽ��2CH3CH2OH��O2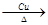 2CH3CHO��2H2O��
2CH3CHO��2H2O��
��5����Ӧ������ϩ�ļӾ۷�Ӧ����Ӧ�Ļ�ѧ����ʽ�� nCH2��CH2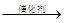
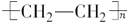 ��
��
��6����ϩ����̼̼˫���������������ӳɷ�Ӧ�������飬a��ȷ�����廯�ⷢ���ӳɷ�Ӧ���������飬c��ȷ��ѡ��b�е��л���������ԭ������ͬһ��̼ԭ���ϣ�������������ϩ�����ӳɷ�Ӧ�õ�����ѡac��
���㣺�����л�����ƶϡ��л���Ӧ���͡������ŵ��ж��Լ�����ʽ����д
�����������ǻ���������Ŀ��飬�漰ϩ���������������������ת�����ѶȲ����Ƕ��л�֪ʶ���ۺ����á��л��ƶ��Ǹ߿��еij������ͣ�ÿ��߿��бؿ�����������ij��Ӧ��ϢҪ��ѧ������Ӧ�ã��ܽϺõĿ��鿼�����Ķ�����ѧ������˼ά���������ȵ����ͣ���Ҫ�ص����ա�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


| ���� |
| �� |

| Cu |
| �� |
| Cu |
| �� |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


| ���� |
| �� |

| ||
| �� |
| ||
| �� |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡͨ����������ѧ��һ��ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
��ϩ��ʯ���ѽ�������Ҫ�ɷ֣����IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ����ش��������⡣
(1)��ϩ�ĵ���ʽ____________���ṹ��ʽ____________��
(2)����������ϩ���Լ���______(�����)��
A��ϡ���� B��������Ȼ�̼��Һ
C��ˮ D�����Ը��������Һ
(3)���������У�����ͨ����ϩ�ӳɷ�Ӧ�õ�����______(�����)��
A��CH3CH3 B��CH3CHCl2
C��CH3CH2OH D��CH3CH2Br
(4)��֪ 2CH3CHO��O2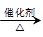 2CH3COOH��������ϩΪ��Ҫԭ�Ϻϳ����ᣬ��ϳ�·������ͼ��ʾ��
2CH3COOH��������ϩΪ��Ҫԭ�Ϻϳ����ᣬ��ϳ�·������ͼ��ʾ��
��Ӧ�ڵĻ�ѧ����ʽΪ____________________________________��
��ҵ������ϩΪԭ�Ͽ�������һ����Ҫ�ĺϳ��л��߷��ӻ�����䷴Ӧ�Ļ�ѧ����ʽΪ_________________ ________________����Ӧ������______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������ʡ�����ѧ��һ��ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
��ϩ��ʯ���ѽ�������Ҫ�ɷ֣����IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ����ش��������⡣
(1)��ϩ�ĵ���ʽ_____________��
(2)����������ϩ���Լ���______(�����)��
| A��ϡ���� | B��������Ȼ�̼��Һ |
| C��ˮ | D�����Ը��������Һ |
 2CH3COOH��������ϩΪ��Ҫԭ�Ϻϳ����ᣬ��ϳ�·������ͼ��ʾ��
2CH3COOH��������ϩΪ��Ҫԭ�Ϻϳ����ᣬ��ϳ�·������ͼ��ʾ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com