
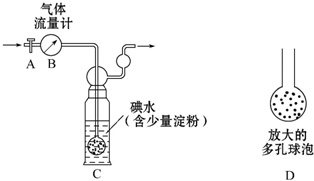
���� �������õ�ԭ��Ϊ��SO2+I2+2H2O=H2SO4+2HI���ݴ�ȷ��������������ʵ�����������β���ж�������ĺ�����
��1��ϴ��ƿC�е���ĩ������һ���������D����������SO2���ˮ�ĽӴ������ʹSO2�͵�ˮ��ַ�Ӧ��
��2��ϴ��ƿC�е���Һ�����������Ը��������Һ����ˮ�ȴ��棻
��3��ϴ��ƿC����Һ��ɫ��ʧ��û�м�ʱ�رջ���A����ͨ��β�����������
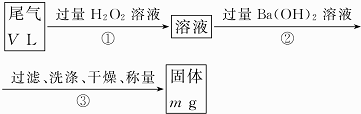
�ҷ������õ�ԭ��Ϊ��SO2+H2O2=H2SO4��H2SO4+Ba��OH��2=BaSO4��+2H2O�����������ᱵ����������β���ж����������������������β��������������
��4�������������������������Ӧ�������ᱵ��ˮ��
��5��ϴ�ӳ����ķ����ǣ���©����ע������ˮ��ʹˮ��û���������ˮ�������ظ�����2��3�Σ�
��6��mg�����ᱵ���������������ᱵ�����ʵ�����������Ԫ���غ������������������������������������������
��7�����ҷ����в����ʡ�ԣ�ֱ�ӽ�β��ͨ�����Ba��OH��2��Һ��BaSO3����������ΪBaSO4��
��� �⣺�������õ�ԭ��Ϊ��SO2+I2+2H2O=H2SO4+2HI�����ⶨʣ������������������β���ж�������ĺ�����
��1��ϴ��ƿC�е���ĩ������һ���������D����������SO2���ˮ�ĽӴ������ʹSO2�͵�ˮ��ַ�Ӧ��
�ʴ�Ϊ������SO2���ˮ�ĽӴ������ʹSO2�͵�ˮ��ַ�Ӧ��
��2��ϴ��ƿC�е���Һ�����������Ը��������Һ����ˮ�ȴ��棻
�ʴ�Ϊ�����Ը��������Һ����ˮ��
��3��ϴ��ƿC����Һ��ɫ��ʧ��û�м�ʱ�رջ���A����ͨ��β��������������SO2����ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
�ҷ������õ�ԭ��Ϊ��SO2+H2O2=H2SO4��H2SO4+Ba��OH��2=BaSO4��+2H2O�����������ᱵ����������β���ж����������������������β��������������
��4��������з�Ӧ�Ļ�ѧ����ʽΪ��H2SO4+Ba��OH��2=BaSO4��+2H2O���ʴ�Ϊ��H2SO4+Ba��OH��2=BaSO4��+2H2O��
��5��ϴ�ӳ����ķ����ǣ���©����ע������ˮ��ʹˮ��û���������ˮ�������ظ�����2��3�Σ�
�ʴ�Ϊ����©����ע������ˮ��ʹˮ��û���������ˮ�������ظ�����2��3�Σ�
��6��mg�����ᱵ�����������ᱵ�����ʵ���Ϊ$\frac{mg}{233g/mol}$=$\frac{m}{233}$mol��������Ԫ���غ��֪������������Ϊ$\frac{m}{233}$mol��22.4L/mol=$\frac{22.4m}{233}$L����β���ж���������������$\frac{\frac{22.4m}{233}}{VL}$=$\frac{22.4m}{233V}$��
�ʴ�Ϊ��$\frac{22.4m}{233V}$��
��7�����ҷ����в����ʡ�ԣ�ֱ�ӽ�β��ͨ�����Ba��OH��2��Һ��BaSO3����������ΪBaSO4�����²ⶨ�������ᱵ������ƫ�ⶨ������������ƫ���������ƫ�ʲ�������
�ʴ�Ϊ����������BaSO3����������ΪBaSO4��
���� ���⿼��ѧ����ʵ��ԭ����ʵ����������⡢ʵ�鷽����ơ�Ԫ�ػ��������ʡ���ѧ����ȣ��Ѷ��еȣ����ʵ��ԭ���ǽ���Ĺؼ�����Ҫѧ���߱���ʵ�Ļ���֪ʶ���ۺ�����֪ʶ�������⡢��������������


 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
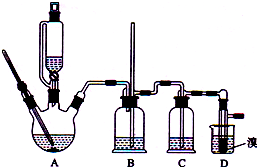 ʵ�����Ʊ�1��2-��������ķ�Ӧԭ�����£�
ʵ�����Ʊ�1��2-��������ķ�Ӧԭ�����£�| �Ҵ� | 1��2-�������� | ���� | |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶ�/g•cm-3 | 0.79 | 2.2 | 0.71 |
| �е�/�� | 78.5 | 132 | 34.6 |
| �۵�/�� | һl30 | 9 | -1l6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | m+n��p | B�� | ƽ�����淴Ӧ�����ƶ� | ||
| C�� | C������������� | D�� | A��ת���ʽ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
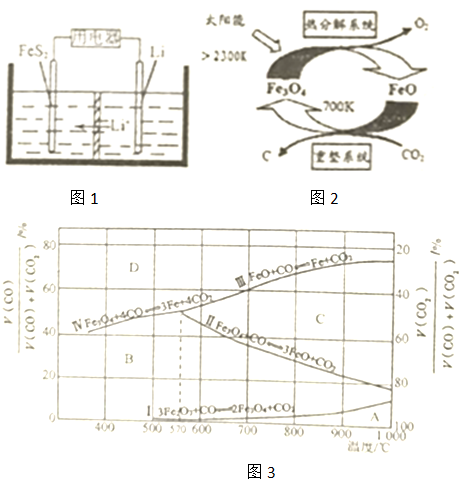
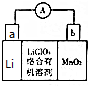 ﮼���ξ��е��������ܺ�����ܣ��ڻ��������ӡ�������ܡ���Դ�����õ��㷺Ӧ�ã�����Ԫ�ر���Ϊ����ԴԪ�ء�����ش��������⣺
﮼���ξ��е��������ܺ�����ܣ��ڻ��������ӡ�������ܡ���Դ�����õ��㷺Ӧ�ã�����Ԫ�ر���Ϊ����ԴԪ�ء�����ش��������⣺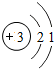 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | K�� | B�� | L�� | C�� | M�� | D�� | N�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ���������� | ���� | |
| A | ��װ��Fe��NO3��2��Һ���Թ��м���ϡH2SO4���ڹܿڹ۲쵽����ɫ���� | HNO3�ֽ�������NO2 |
| B | �������Һ�м���ϡH2SO4�����ȼ����ӣ���ȴ���ټ�������Cu��OH��2��Һ�����ȣ�û�к�ɫ�������� | ����û��ˮ��������� |
| C | ��Na2CO3��Һ��ͨ������CO2����Һ����� | ������NaHCO3 |
| D | ����ˮ�Ҵ��м���ŨH2SO4��������170�����������ͨ������KMnO4��Һ����ɫ��ȥ | ʹ��Һ��ɫ����������ϩ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2.24LNH3��CH4�Ļ�����壬������������Ϊ2NA | |
| B�� | 1mol�Ҵ���CH3CH2OH�������к��й��ۼ�����Ϊ8NA | |
| C�� | 1mol/LNa2CO3��Һ�У�����CO32-������ΪNA | |
| D�� | 6.4gCu������Ũ���ᷴӦ������ˮ���ռ������������Ϊ0.2NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������������Ʒ���Թܵ�����ˮ���У��Թ���Һ����������ԭ������Ʒ��һ����NH3 | |
| B�� | �������ǵ�ľ������ʢ��������Ʒ�ļ���ƿ�У�ľ��δ��ȼ����ԭ������Ʒ��һ������O2 | |
| C�� | ��һ�����������Ʒ����ͨ�����ʯ��ˮ�У�δ���а�ɫ��������ԭ������Ʒ��һ������CO2 | |
| D�� | ��һ�����������Ʒ����ͨ��ˮ�У�ǡ����ȫ��Ӧ����һ���Σ�����������Һ�м������ʯ��ˮ��δ���������ɣ���ԭ������Ʒ��V��NH3����V��O2��=4��1 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com