
 =0.02mol��������Һ������ԭ���֪��Һ�л�Ӧ�� K+��Mg2+�����е�����һ�֣�
=0.02mol��������Һ������ԭ���֪��Һ�л�Ӧ�� K+��Mg2+�����е�����һ�֣� =0.02mol����c��SO42-��=
=0.02mol����c��SO42-��=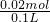 =0.2mol/L���ʴ�Ϊ��0.2 mol/L��
=0.2mol/L���ʴ�Ϊ��0.2 mol/L��

 ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012�꽭��ʡ����һ�и�һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��10�֣���1����ָ�����������зֱ�Ӧ������Щ���뷽���������ˡ����������� ����ȡ���ᾧ��
a��������ҩ__________________ b�����붹���Ͷ���__________________
c���ú�ˮɹ��_________________ d����ˮ����__________________
(2 )����һ������ˮ��Һ��ֻ���ܺ������������е������֣�K����H����M g2����Ba2����CO32����SO42������ȡ����100mL��Һ��������ʵ�飺
g2����Ba2����CO32����SO42������ȡ����100mL��Һ��������ʵ�飺
�ٵ�һ�ݼ�������NaHCO3��Һ���ռ�������0.03mol��
�ڵڶ��ݼ�����Ba(NO3)2��Һ��ַ�Ӧ����˸���ø������4.66g��
��������ʵ��ش�
��a ��ԭ��Һ��һ�������ڵ�������_______________________��
��ԭ��Һ��һ�������ڵ�������_______________________��
��b��ԭ��Һ�п��ܴ��ڵ�������_______________________��
��c��������ԭ��Һ��һ�����ڵ������ӵ����ʵ���Ũ��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012�꽭��ʡ��һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��10�֣���1����ָ�����������зֱ�Ӧ������Щ���뷽���������ˡ����������� ����ȡ���ᾧ��
a��������ҩ__________________ b�����붹���Ͷ���__________________
c���ú�ˮɹ��_________________ d����ˮ����__________________
(2 )����һ������ˮ��Һ��ֻ���ܺ������������е������֣�K����H����Mg2����Ba2����CO32����SO42������ȡ����100mL��Һ��������ʵ�飺
�ٵ�һ�ݼ�������NaHCO3��Һ���ռ�������0.03mol��
�ڵڶ��ݼ�����Ba(NO3)2��Һ��ַ�Ӧ����˸���ø������4.66g��
��������ʵ��ش�
��a��ԭ��Һ��һ�������ڵ�������_______________________��
��b��ԭ��Һ�п��ܴ��ڵ�������_______________________��
��c��������ԭ��Һ��һ�����ڵ������ӵ����ʵ���Ũ��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010������ʡ��һ��ѧ�����п��ԣ���ѧ�� ���ͣ������
��1����ָ�����������зֱ�Ӧ������Щ���뷽���������ˡ����������� ����ȡ���ᾧ��
a��������ҩ__________________ b�����붹���Ͷ���__________________
c���ú�ˮɹ��____________________ d����ˮ����__________________
��2��д�����ӷ�Ӧ����ʽH+ + OH- =H2O����Ӧ��������ͬ���ͻ�ѧ����ʽ
��
��
��3���������з�Ӧ�У�
A. 2F2+2H2O=4HF+O2 B. 2Na+2H2O=2NaOH+H2��
C. CaO+H2O=Ca(OH)2 D. 2H2O=2H2��+O2��
ˮֻ������������ ��ˮֻ����ԭ������ ��ˮ����������������ԭ������ ��ˮ�Ȳ����������ֲ�����ԭ������ ���������ű�ʾB��Ӧ�ĵ���ת�Ʒ������Ŀ��
2Na+2H2O=2NaOH+H2��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com