
��ͼ�׳غ��ҳ��е��ĸ��缫���Dz��缫���ҳ���Һ�ֲ㣬�ϲ���ҺΪ����Һ�������ԡ������ͼʾ�ж������й�˵����ȷ���ǣ�
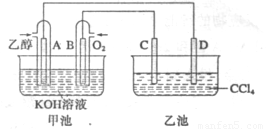
A���׳صĵ��أ��ҳ���ԭ���
B��ͨ���Ҵ��IJ��缫��ӦʽΪC2H5OH+16OH-+12e-=2CO32-+11H2O
C����Ӧһ��ʱ���������Һ��pH��δ�仯
D�������ҳ��м���NaI��Һ�������ҳط�Ӧ�����У����Թ۲쵽C�缫��Χ����Һ�����ػ�ɫ����Ӧ��Ϻ��ò�����������Һ�����²���Һ�����Ϻ�ɫ���ϲ�ӽ���ɫ
D
��������
�����������ͼ��֪�׳����Ҵ�ȼ�ϵ�أ���ԭ��أ��ҳ����ǵ��أ�A������KOH�����Һ�������£������ط�Ӧ���ܷ�ӦʽΪ��C2H5OH+3O2+4KOH=2K2CO3+5H2O��������ӦΪ��3O2+6H2O+12e-=12OH-����ʽ����ɵ�������Ӧʽ��B���׳����Ҵ�ȼ�ղ�����CO2����KOH��Ӧ����Һ��pH�����ı䣬C����C��ԭ��������������ǵ��ص�������I-�ŵ磬����I2����ɫ��Һ����CCl4��ȡ���²�Ϊ�л�������ɫ���ϲ�Ϊˮ�����ɫ��D��ȷ��
���㣺ԭ��ء�����ԭ�����缫��Ӧʽ����д��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ������������������ѧ�߶����п��Ի�ѧ�Ծ����������� ���ͣ������
��8�֣���ͼ�׳غ��ҳ��е��ĸ��缫���Ƕ��Բ��ϣ��ҳ���Һ�ֲ㣬�²�Ϊ���Ȼ�̼���ϲ���ҺΪ����Һ�������ԣ������ͼʾ�ش��������⣺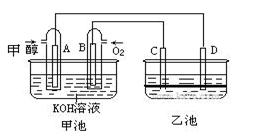
(1)ͨ��״��Ķ��Ե缫�ĵ缫��ӦʽΪ �����׳ؿ��Գ�磬���ʱA�ӵ�Դ�ĸ�������ʱB�������ĵ缫��ӦʽΪ ��
(2)���ҳط�Ӧ�����У����Թ۲쵽 �缫��Χ����Һ�����غ�ɫ����Ӧ��Ϻ��ò�����������Һ�����²���Һ�����Ϻ�ɫ���ϲ�ӽ���ɫ�� C�������ĵ缫��ӦʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���㽭ʡ������ѧ�߶���ѧ�ڵ�һ���¿���ѧ�Ծ����������� ���ͣ������
��ͼ�׳غ��ҳ��е��ĸ��缫���Ƕ��Բ��ϣ��ҳ���Һ�ֲ㣬�²�Ϊ���Ȼ�̼���ϲ���ҺΪ����Һ�������ԣ������ͼʾ�ش��������⣺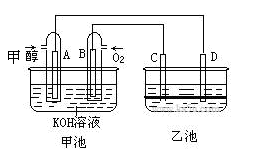
(1)ͨ��״��Ķ��Ե缫�ĵ缫��ӦʽΪ �����׳ؿ��Գ�磬���ʱA�ӵ�Դ�ĸ�������ʱB�������ĵ缫��ӦʽΪ ��
(2)���ҳط�Ӧ�����У����Թ۲쵽 �缫��Χ����Һ�����غ�ɫ����Ӧ��Ϻ��ò�����������Һ�����²���Һ�����Ϻ�ɫ���ϲ�ӽ���ɫ�� C�������ĵ缫��ӦʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
��9�֣���ͼ�׳غ��ҳ��е��ĸ��缫���Ƕ��Բ��ϣ��ҳ���Һ�ֲ㣬�ϲ���ҺΪ����Һ�������ԣ������ͼʾ�ش��������⣺
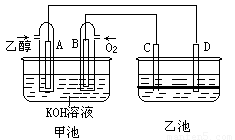
(1)ͨ���Ҵ���C2H5OH���Ķ��Ե缫�ĵ缫��ӦʽΪ ��
���׳ؿ��Գ�磬���ʱA�ӵ�Դ�ĸ�������ʱB�������ĵ缫��ӦʽΪ ��
(2)���ҳط�Ӧ�����У����Թ۲쵽 �缫��Χ����Һ�����غ�ɫ����Ӧ��Ϻ��ò�����������Һ�����²���Һ�����Ϻ�ɫ���ϲ�ӽ���ɫ��C�������ĵ缫��Ӧʽ ��
(3)���ڳ��³�ѹ�£�1gC2H5OHȼ������CO2��Һ̬H2Oʱ�ų�29.71kJ��������ʾ�÷�Ӧ���Ȼ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������ʡ�����и߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��8�֣���ͼ�׳غ��ҳ��е��ĸ��缫���Ƕ��Բ��ϣ��ҳ���Һ�ֲ㣬�²�Ϊ���Ȼ�̼���ϲ���ҺΪ����Һ�������ԣ������ͼʾ�ش��������⣺
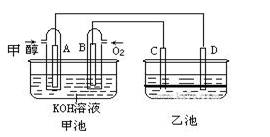
(1)ͨ��״��Ķ��Ե缫�ĵ缫��ӦʽΪ �����׳ؿ��Գ�磬���ʱA�ӵ�Դ�ĸ�������ʱB�������ĵ缫��ӦʽΪ ��
(2)���ҳط�Ӧ�����У����Թ۲쵽 �缫��Χ����Һ�����غ�ɫ����Ӧ��Ϻ��ò�����������Һ�����²���Һ�����Ϻ�ɫ���ϲ�ӽ���ɫ�� C�������ĵ缫��ӦʽΪ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com