

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �����ڳ�ȥ���ٹ�·��ѩ���ǡ������ࡱ��ѩ������NaCl��MgCl2�ȣ���ش�
�����ڳ�ȥ���ٹ�·��ѩ���ǡ������ࡱ��ѩ������NaCl��MgCl2�ȣ���ش�| ������/kJ?mol-1 | I1 | I2 | I3 | I4 |
| X | 578 | 1817 | 2745 | 11578 |
| Y | 738 | 1451 | 7733 | 10540 |
| Z | 496 | 4562 | 6912 | 9543 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
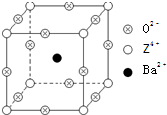 ��2011?������ģ����֪X��Y��Z����Ԫ�ص�ԭ������֮�͵���48��X��һ��1��1���⻯������м��ЦҼ����Цм���Z�ǽ���Ԫ�أ�Z�ĵ��ʺͻ������й㷺����;����֪Z�ĺ˵����С��28���Ҵ������2��δ�ɶԵ��ӣ���ҵ������ZO2��̼�ᱵ������״̬����ȡ������M��M�ɿ���һ�ֺ������Σ���M�������ġ�ѹ�����ܡ���Ӧ���ڳ������ķ���װ�ã���X���߷�����M�������С�ظ���λΪ�����壨��ͼ�����߳�Ϊ4.03��10-10m������λ��ΪZ4+��ռ������λ��ΪBa2+��ռ����������λ��ΪO2-��ռ��
��2011?������ģ����֪X��Y��Z����Ԫ�ص�ԭ������֮�͵���48��X��һ��1��1���⻯������м��ЦҼ����Цм���Z�ǽ���Ԫ�أ�Z�ĵ��ʺͻ������й㷺����;����֪Z�ĺ˵����С��28���Ҵ������2��δ�ɶԵ��ӣ���ҵ������ZO2��̼�ᱵ������״̬����ȡ������M��M�ɿ���һ�ֺ������Σ���M�������ġ�ѹ�����ܡ���Ӧ���ڳ������ķ���װ�ã���X���߷�����M�������С�ظ���λΪ�����壨��ͼ�����߳�Ϊ4.03��10-10m������λ��ΪZ4+��ռ������λ��ΪBa2+��ռ����������λ��ΪO2-��ռ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����ѧ--ѡ��3�����ʽṹ�����ʡ�
����ѧ--ѡ��3�����ʽṹ�����ʡ�



| ||
| ||
| 233g/mol |
| (4.03��10-8 cm)3NA |
| 233g/mol |
| (4.03��10-8 cm)3NA |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com