
��16�֣�ij�����A������KAl(SO4)2��Al2O3��Fe2O3����һ�������¿�ʵ����ͼ��ʾ������֮��ı仯��

�ݴ˻ش��������⣺
��1��I��II��III��IV�IJ��ж�����Һ�ͳ����ķ����ȡ�ķ����� ��
��2������������ͼ��Ӧ��ϵ��д������B��C��D��E�������ʵĻ�ѧʽ����B ������C ������D ��
��3��д���١��ڡ���������Ӧ����ʽ���������ӷ�Ӧ��д���ӷ���ʽ��
�� ��
�� ��
�� ��
��1������ ��2��Al2O3 Al2O3��Fe2O3 Fe2O3
��3����Al2O3��2NaOH��2NaAlO2��H2O ��Al3+��3NH3��H2O��Al(OH) 3����3NH4+
��2Al(OH) 3 Al2O3��3H2O
Al2O3��3H2O
��������
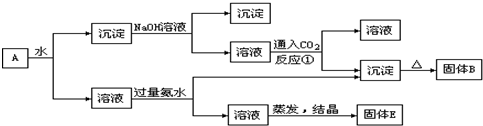
���������KAl(SO4)2����ˮ��Al2O3��Fe2O3������ˮ������A��ˮ����ҺΪKAl(SO4)2��Һ������CΪAl2O3��Fe2O3��Al2O3��NaOH��Һ��Ӧ�����Գ���C��NaOH��Һ��õ���ҺΪNaAlO2��Һ������DΪFe2O3��NaAlO2��Һ���������ᷴӦ����Al(OH)3���������Ⱥ�õ��Ĺ���BΪAl2O3��KAl(SO4)2��Һ�м�������İ�ˮ��õ�����Al(OH)3��
���㣺����ͼ
��������ѧ�ƶ�����һ���ۺ��Խ�ǿ������,��Ԫ�ؼ����������ʺ��������,��������,��ѧ�����֪ʶ,��������ѧ�Ƽ��ۺ�.�������ɿ���ѧ���Ի�ѧ֪ʶ������̶�,����Ҫ��������ѧ�����ۺϷ���������˼ά����.�����ͼ��ķ���:��ؼ�����Ѱ��"ͻ�ƿ�"!��"ͻ�ƿ�"����ץ"��"�֣�����������ɫ������״̬��������ζ�����ⷴӦ���������������Ʒ���������;�ȡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�ݴ��жϣ�
��1������B�������ʵĻ�ѧʽΪ_______________��
��2������E�������ʵĻ�ѧʽΪ_______________________��
��3����Ӧ�ٵ����ӷ���ʽΪ_______________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com