
| ����� | ƽ�ⷽ��ʽ | ƽ�ⳣ��K | Ksp |
| CH3COOH | CH3COOH CH3COO-��H+ CH3COO-��H+ | 1.76��10-5 | |
| H2CO3 | H2CO3 H+��HCO3- H+��HCO3-HCO3-  H+��CO32- H+��CO32- | K1��4.31��10-7 K2��5.61��10-11 | |
| C6H5OH | C6H5OH  C6H5O-��H+ C6H5O-��H+ | 1.1��10-10 | |
| H3PO4 | H3PO4 H+��H2PO4- H+��H2PO4-H2PO4-  H+��HPO32- H+��HPO32-HPO42-  H+��PO43- H+��PO43- | K1��7.52��10-3 K2��6.23��10-8 K3��2.20��10-13 | |
| NH3��H2O | NH3��H2O NH4+��OH- NH4+��OH- | 1.76��10-5 | |
| BaSO4 | BaSO4 Ba2+��SO42- Ba2+��SO42- | | 1.07��10-10 |
| BaCO3 | BaCO3 Ba2+��CO32- Ba2+��CO32- | | 2.58��10-9 |
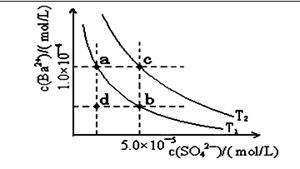
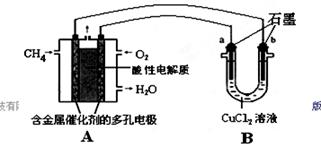
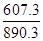 =0.7�� ��2���ٵ����Ϊ���Թʸ�����ӦʽΪ��CH4-8e-+2H2O=CO2+8H+ ��Cu2+�������ŵ缴b����ÿͨ��0.1mol���ӣ���0.5molCu2+�ŵ磬��3.2g �棨1�� KֵԽ������Խǿ ��2��25��ʱ����Ͱ�ˮ�ĵ���̶���ͬ���ʽ��������Ũ�ȵĴ���Ͱ�ˮ��Ϻ���Һ�����ԣ��ʻ��Һ��c(CH3COO-)=c(NH4+)����3����ΪNH3��H2O�ĵ���̶�Զ����C6H5OH������10ml 0.01mol/L������Һ�еμ�Vml 0.01mol/L��ˮ����ˮ���������Ҫ��10mL��Һ��pH>7,A ����B����ѭ����غ㣬����V=10ʱ�����ҺΪ�������Һ�������ˮ��ٽ�ˮ�ĵ��룬�ʻ��Һ��ˮ�ĵ���̶ȴ���10ml 0.01mol/L������Һ��ˮ�ĵ���̶ȣ�C����4�������¶ȣ�BaSO4�ĵ���ƽ�������ƶ���c��SO42-����c(Ba2+)������D����
=0.7�� ��2���ٵ����Ϊ���Թʸ�����ӦʽΪ��CH4-8e-+2H2O=CO2+8H+ ��Cu2+�������ŵ缴b����ÿͨ��0.1mol���ӣ���0.5molCu2+�ŵ磬��3.2g �棨1�� KֵԽ������Խǿ ��2��25��ʱ����Ͱ�ˮ�ĵ���̶���ͬ���ʽ��������Ũ�ȵĴ���Ͱ�ˮ��Ϻ���Һ�����ԣ��ʻ��Һ��c(CH3COO-)=c(NH4+)����3����ΪNH3��H2O�ĵ���̶�Զ����C6H5OH������10ml 0.01mol/L������Һ�еμ�Vml 0.01mol/L��ˮ����ˮ���������Ҫ��10mL��Һ��pH>7,A ����B����ѭ����غ㣬����V=10ʱ�����ҺΪ�������Һ�������ˮ��ٽ�ˮ�ĵ��룬�ʻ��Һ��ˮ�ĵ���̶ȴ���10ml 0.01mol/L������Һ��ˮ�ĵ���̶ȣ�C����4�������¶ȣ�BaSO4�ĵ���ƽ�������ƶ���c��SO42-����c(Ba2+)������D����


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��25����ˮ�����c��H+��= 10-12mol/L ����Һ��Fe3+ Cl��NO3�� K+ |
| B��ʹ��̪������Һ��Na+ Cl�� SO42��AlO2�� |
| C��ij��ɫ��Һ��HCO3��NO3�� Al3+ Ba2+ |
| D��25��ʱ��pH=1����Һ�� Ba2+ NO3�� K+ I�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
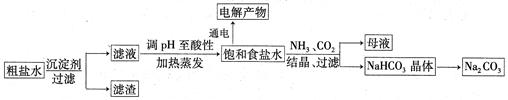
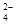 ���õ�������NaCl���壬����������Լ���
���õ�������NaCl���壬����������Լ���| A��������NaOH��Һ�� | B��������Na2CO3��Һ�� | C����������� | D��������BaCl2��Һ�� |
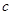 ��OH-���ı�ֵ��_________��
��OH-���ı�ֵ��_________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������Һ�У�Na+��K+��SO32����S2�� |
| B����ɫ��Һ�У�Na+��Fe3+��Cl����SO42�� |
| C����ɫ��Һ�У�Na+��K+��HCO3����AlO2�� |
| D��������Һ�У�Na+��K+��NO3����ClO�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Fe3+��K+��Cl-��NO3- | B��Ag+��Na+��NO3-��Cl- |
| C��Zn2+��Al3+��SO42-��Cl- | D��Ba2+��NH4+��Cl-��HCO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ˮ�У�NH4����Fe2����SO42����HSO3�� |
| B��pH=l����Һ�У���Mg2����Fe3����Cl����NO3�� |
| C��ͨ������CO2����Һ�У�Ca2����Na����SiO32����CO32�� |
| D����ˮ�������c(OH��)��1��10��12mol��L��1����Һ�У�NH4+��HCO3����Na+��Cl�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ���� | ������ | ���ӹ����жϼ���Ӧ�����ӷ���ʽ |
| A | �μӰ�ˮ | Na����Al3����Cl����NO3�� | ���ܹ��棬Al3����3OH����Al(OH)3�� |
| B | pH��1����Һ | Fe2����Al3����SO42����MnO4�� | ���ܹ��棬 5Fe2����MnO4����8H����Mn2����5Fe3����4H2O |
| C | ��ˮ�������H��Ũ��Ϊ1��10��12mol/L | NH4����Na����NO3����Cl�� | һ�����棬NH4����H2O NH3��H2O��H�� NH3��H2O��H�� |
| D | ͨ������SO2���� | K����Na����ClO����SO42�� | ���ܹ��棬 2ClO����SO2��H2O��2HClO��SO32�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������£�������Һ�У�������Һ��pH=1������������Һ��pH=13 |
| B�������£�������Һ��Ϻ�pH=7������Һ��c(Na��)��c(CH3COO��) |
| C���� V1= V2��������Һ��ϣ����û��Һ��c(CH3COO��)��c(Na��)��c(H��)��c(OH��) |
| D��V1��V2����Ȼ�ϣ����û��Һ��c (Na��)+ c(H+) ��c(OH��)+ c (CH3COO��) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��1.0 mol��L��1��KNO3��Һ��H����Fe2����Cl����SO42�� |
| B��������ˮ�У�NH4����SO32����AlO2����Cl�� |
| C��������Ӧ����������������Һ��Na����K����CO32����NO3�� |
| D��c(H��)=1.0��10��13mol/L��Һ�У�K����Na����CH3COO����Br�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com