
��13�֣��ס�����ͬѧ����ȡ������Fe(OH)2 ��������ͼ��ʾ��װ�ý������顣A������Fe+H2SO4 �� B������NaOH��Һ����ش��������⡣

A B
��1��ͬѧ�ף��ȼн�ֹˮ��a��ʹA�ܿ�ʼ��Ӧ����B���й۲쵽�������� ��
��2��ͬѧ�ң���a��ʹA���з�Ӧһ��ʱ���ټн�ֹˮ��a��ʵ������B���й۲쵽�������� ��
B�з�����Ӧ�����ӷ���ʽΪ
��3��ͬѧ�Ҵ�a��Ŀ�� �� ������ң�ͬѧ�ɳɹ���
��1����3�֣���B���й۲쵽�������ǣ����ɻҰ�ɫ����Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ��
��2����6�֣��а�ɫ�������� Fe2++2OH-=Fe(OH)2¯
��3����2�֣�ʹA���в���������ͨ��B���и��߿��� ��2�֣���
������������Fe(OH)2 �ױ������е������������������Ʊ����ؼ��Ǹ���������
��1���ȼн�ֹˮ��a��ʹA�ܿ�ʼ��Ӧ����������������ӦҺ��FeCl2��ѹ���Թ�B��FeCl2��NaOH��Һ��Ӧ�õ�Fe(OH)2��ɫ��������ϵͳ�������Ĵ��ڻὫ�����������ɻҰ�ɫ����Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ
��2����a��ʹA���з�Ӧһ��ʱ�䣬�����������ܶ�С��������Թ�B�У���ϵͳ�е������ų����ټн�ֹˮ��a����������������ӦҺ��FeCl2��ѹ���Թ�B��FeCl2��NaOH��Һ��Ӧ�õ�Fe(OH)2��ɫ���������ҿ��ȶ����ڡ�
��3�������ԣ���ͬѧ���Ʒ��ɳɹ��ﵽʵ��Ŀ�ġ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ף�NaOH��Һ![]() Na2CO3��Һ
Na2CO3��Һ
�ң�NaOH��Һ![]() NaHCO3��Һ
NaHCO3��Һ![]()
Na2CO3����![]() Na2CO3��Һ
Na2CO3��Һ
����NaOH��Һ![]() NaHCO3��Һ
NaHCO3��Һ![]()
������������⣺
��1����ָ���ס�������������Ҫȱ�㣺
����_________________________________��
�ҷ���_________________________________��
��2�������ñ�������ȡ��Na2CO3��Һ����ʵ��Ĺؼ���______________________��
��3�����跴Ӧ�����õ�NaHCO3��Һ�к���Na2CO3����Ҫ����A��D�����Լ�֤��Na2CO3�Ĵ��ڣ�����ѡ����Լ���___________�����Լ����ţ���
A.����ʯ��ˮ B.CaCl2��Һ
C.Ba(NO3)2 D.����
��4�����跴Ӧ�����õ�Na2CO3�����л���δ�ֽ��NaHCO3����ͬѧѡ�����ʯ��ˮ�ⶨ����NaHCO3��������������ͬѧ�ܷ�ﵽʵ��Ŀ�ģ�___________����ܡ����ܡ��������ܣ���˵���������Ҫ�����ݣ������ܣ����Ҫ˵�����ɡ�
__________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ף�NaOH��Һ![]() Na2CO3��Һ
Na2CO3��Һ

������������⣺
��1����ָ���ס�������������Ҫȱ�㣺
����_______________________________________��
�ҷ���_______________________________________��
��2�������ñ�������ȡ��Na2CO3��Һ����ʵ��Ĺؼ���________________��
��3�����跴Ӧ�����õ�NaHCO3��Һ�к���Na2CO3����Ҫ����A��D�����Լ�֤��Na2CO3�Ĵ��ڣ�����ѡ����Լ���_________________(���Լ�����������У���
��4�����跴Ӧ�����õ�Na2CO3�����л���δ�ֽ��NaHCO3����ͬѧѡ�����ʯ��ˮ�ⶨ����NaHCO3��������������ͬѧ�ܷ�ﵽʵ��Ŀ�ģ�����ܡ����ܡ��������ܣ���˵���������Ҫ�����ݣ������ܣ����Ҫ˵�����ɡ�
_____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A.����ʯ B.���� C.����������Һ D.����ʯ��ˮ
����ͬѧ��Ƶ��Ʊ�ʵ�鷽���ķ�Ӧ���̷ֱ����£�
�ף�NaOH��Һ![]() Na2CO3��Һ
Na2CO3��Һ

������������⣺
��1����ָ���ס�������������Ҫȱ�㣺
����_____________________________________________________________��
�ҷ���_____________________________________________________________��
��2�������ñ�������ȡ��Na2CO3��Һ����ʵ��Ĺؼ���_____________________________��
��3�����跴Ӧ�����õ�NaHCO3��Һ�к���Na2CO3����Ҫ����A��D�����Լ�֤��Na2CO3�Ĵ��ڣ�����ѡ����Լ���___________�����Լ����ţ���
��4�����跴Ӧ�����õ�Na2CO3�����л���δ�ֽ��NaHCO3����ͬѧѡ�����ʯ��ˮ�ⶨ����NaHCO3��������������ͬѧ�ܷ�ﵽʵ��Ŀ�ģ�___________����ܡ����ܡ������ܣ���˵���������������ݣ������ܣ����Ҫ˵�����ɣ�___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧ����С������ȡ��Na2CO3��Һ������ʵ������е�������⡣�ɹ�ѡ����Լ��У�A������ʯ B������ C������������Һ D������ʯ��ˮ������ͬѧ��Ƶ��Ʊ�ʵ�鷽���ķ�Ӧ���̷ֱ����£�
�ף�NaOH��Һ![]() Na2CO3��Һ
Na2CO3��Һ
�ң�NaOH��Һ![]() NaHCO3��Һ
NaHCO3��Һ![]() Na2CO3����
Na2CO3����![]() Na2CO3��Һ
Na2CO3��Һ
����NaOH��Һ![]() NaHCO3��Һ
NaHCO3��Һ![]() Na2CO3��Һ
Na2CO3��Һ
������������⣺
��1����ָ���ס�������������Ҫȱ�㣺
����_______________________________________��
�ҷ���_______________________________________��
��2�������ñ�������ȡ��Na2CO3��Һ����ʵ��Ĺؼ���________________��
��3�����跴Ӧ�����õ�NaHCO3��Һ�к���Na2CO3����Ҫ����A��D�����Լ�֤��Na2CO3�Ĵ��ڣ�����ѡ����Լ���_________________(���Լ�����������У���
��4�����跴Ӧ�����õ�Na2CO3�����л���δ�ֽ��NaHCO3����ͬѧѡ�����ʯ��ˮ�ⶨ����NaHCO3��������������ͬѧ�ܷ�ﵽʵ��Ŀ�ģ�����ܡ����ܡ��������ܣ���˵���������Ҫ�����ݣ������ܣ����Ҫ˵�����ɡ�
_____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���˽̰���л�ѧѡ��6 2.2 ���ʵ��Ʊ���ϰ���������棩 ���ͣ������
ij��ѧ����С������ȡ��Na2CO3��Һ������ʵ������е�������⡣�ɹ�ѡ����Լ���
A������ʯ B������
C������������Һ D������ʯ��ˮ
����ͬѧ��Ƶ��Ʊ�ʵ�鷽���ķ�Ӧ���̷ֱ����£�
�ף�NaOH��Һ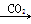 Na2CO3��Һ
Na2CO3��Һ
�ң�NaOH��Һ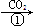 NaHCO3��Һ
NaHCO3��Һ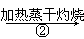 Na2CO3����
Na2CO3����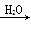 Na2CO3��Һ
Na2CO3��Һ
����NaOH��Һ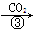 NaHCO3��Һ
NaHCO3��Һ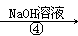 Na2CO3��Һ
Na2CO3��Һ
������������⣺
(1)��ָ���ס�������������Ҫȱ�㣺
����________________________________________________________________________��
�ҷ���________________________________________________________________________��
(2)�����ñ�������ȡ��Na2CO3��Һ����ʵ��Ĺؼ���
________________________________________________________________________��
(3)���跴Ӧ�����õ�Na2CO3�����л���δ�ֽ��NaHCO3����ͬѧѡ�����ʯ��ˮ�ⶨ����NaHCO3��������������ͬѧ�ܷ�ﵽʵ��Ŀ�ģ�________(��ܡ����ܡ�)�����ܣ���˵��������������ݣ������ܣ����Ҫ˵������________________________________________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com