
�������ữ�ĸ��������Һ����������Һ��Ϻ�����Ӧ�Ļ�ѧ����ʽ���£�2KMnO4 + 5H2O2 + 3H2SO4 = 2MnSO4 + K2SO4 + 5O2��+ 8H2O ���жԴ˷�Ӧ�ķ�����ȷ����
A��������������ԭ����������������
B���������������ԭ������������
C��������������ԭ�����������ữ����
D�������������������������������

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
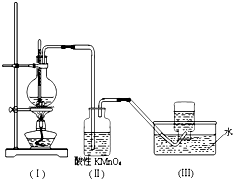 ��ͼ��ʾ����ʵ�����ͨ������Ũ�������Ҵ���ϼ�������ϩ������һ��ʱ���۲쵽��ƿ����Һ��ڣ�װ�ã����о������ữ�ĸ��������Һ��ɫ��
��ͼ��ʾ����ʵ�����ͨ������Ũ�������Ҵ���ϼ�������ϩ������һ��ʱ���۲쵽��ƿ����Һ��ڣ�װ�ã����о������ữ�ĸ��������Һ��ɫ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����һ��ѧ�������¡��л������3.2.1��ϩ(�˽̰����2) ���ͣ�058
��ͼ��ʾ����ʵ�����ͨ������Ũ�������Ҵ���ϼ�������ϩ������һ��ʱ�����Һ������ɫ����ɫ������֣���һ��ʱ����־������ữ�ĸ��������Һ��ɫ����ͬѧ��Ϊ���Ը��������Һ��ɫ����֤����ϩ�����Ը��������Һ�����ˣ���ͬѧ��Ϊ���Ը��������Һ��ɫ������֤����ϩ�����Ը��������Һ�����ˣ�
(1)����Ϊ�ĸ�ͬѧ�Ĺ۵���ȷ��________(��ס����ҡ�)��������________��
A��(��)ƿ�����Ը��������Һ��ɫ����֤����ϩ������������Ӧ
B��(��)ƿ�����Ը��������Һ��ɫ����֤����ϩ�����˼ӳɷ�Ӧ
C��(��)ƿ�����Ը��������Һ��ɫ������֤��ͨ��������Ǵ�����
D��(��)ƿ�����Ը��������Һ��ɫ��ֻ��֤��ͨ�������һ�����л�ԭ��
(2)��ͬѧȡ(��)ƿ��������Һ���Թ������������Ȼ�����Һ��������ɫ����������Ϊ��ϩ��һ�����ж�����������Ϊ���Ľ����Ƿ�ɿ���________(��ɿ������ɿ���)��������________________��
������Ϊ���ɿ����Ľ�����ʵ�鷽����֤����ϩ���Ƿ���SO2��_____________��
(3)��ͬѧ������ʵ�鷽���������ʵ��Ľ�������֤����ϩ�ܷ����ӳɷ�Ӧ�����ĸĽ�������������װ��(��)��(��)֮������һ��װ������________��ϴ��ƿ�ҽ�(��)ƿ����Һ����________��������Ӧ�Ļ�ѧ����ʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������ƺ��ص�һ����ѧ��һ��ѧ�ڵ������¿���ѧ������������ ���ͣ�ʵ����
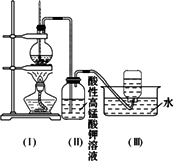
��ͼ��ʾ����ʵ�����ͨ������Ũ�������Ҵ���ϼ�������ϩ: CH3CH2OH CH2=CH2��+H2O������һ��ʱ�����Һ���к�ɫ������֡���һ��ʱ��������ữ�ĸ��������Һ��ɫ����������֪�������������к���CH2=CH2��SO2��CO2��H2O��
CH2=CH2��+H2O������һ��ʱ�����Һ���к�ɫ������֡���һ��ʱ��������ữ�ĸ��������Һ��ɫ����������֪�������������к���CH2=CH2��SO2��CO2��H2O��
���Ը��������Һ��ɫ����ͬѧ��Ϊ��֤����ϩ�����Ը��������Һ�����ˣ���ͬѧ��Ϊ����֤����ϩ�����Ը��������Һ�����ˡ�
��1������Ϊ�ĸ�ͬѧ�Ĺ۵���ȷ�� _____ ����ס����ҡ����������ǣ�������ѡ����ѡ��_____
| A��(��)ƿ�����Ը��������Һ��ɫ����֤����ϩ������������Ӧ |
| B��(��)ƿ�����Ը��������Һ��ɫ����֤����ϩ�����˼ӳɷ�Ӧ |
| C��(��ƿ�����Ը��������Һ��ɫ������֤��ͨ��������Ǵ����� |
| D��(��)ƿ�����Ը��������Һ��ɫ��ֻ��֤��ͨ�������һ�����л�ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015������ƺ��ص�һ����ѧ��һ��ѧ�ڵ������¿���ѧ���������棩 ���ͣ�ʵ����
��ͼ��ʾ����ʵ�����ͨ������Ũ�������Ҵ���ϼ�������ϩ: CH3CH2OH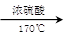 CH2=CH2��+H2O������һ��ʱ�����Һ���к�ɫ������֡���һ��ʱ��������ữ�ĸ��������Һ��ɫ����������֪�������������к���CH2=CH2��SO2��CO2��H2O��
CH2=CH2��+H2O������һ��ʱ�����Һ���к�ɫ������֡���һ��ʱ��������ữ�ĸ��������Һ��ɫ����������֪�������������к���CH2=CH2��SO2��CO2��H2O��
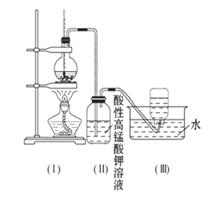
���Ը��������Һ��ɫ����ͬѧ��Ϊ��֤����ϩ�����Ը��������Һ�����ˣ���ͬѧ��Ϊ����֤����ϩ�����Ը��������Һ�����ˡ�
��1������Ϊ�ĸ�ͬѧ�Ĺ۵���ȷ�� _____ ����ס����ҡ����������ǣ�������ѡ����ѡ��_____
A��(��)ƿ�����Ը��������Һ��ɫ����֤����ϩ������������Ӧ
B��(��)ƿ�����Ը��������Һ��ɫ����֤����ϩ�����˼ӳɷ�Ӧ
C��(��ƿ�����Ը��������Һ��ɫ������֤��ͨ��������Ǵ�����
D��(��)ƿ�����Ը��������Һ��ɫ��ֻ��֤��ͨ�������һ�����л�ԭ��
��2����ͬѧȡ����ƿ��������Һ���Թ������������Ȼ�����Һ��������ɫ����������Ϊ��ϩ��һ�����ж�����������Ϊ���Ľ����Ƿ�ɿ��� _____ ����ɿ������ɿ�������������_____________________ ��
��3����ͬѧ��֤����ϩ�ܷ����巢����Ӧ�����Ƕ�����ʵ������˸Ľ����Ľ��ķ����ǣ���װ�ã��ͣ���֮������һ��װ������_____��ϴ��ƿ���ҽ�����ƿ����Һ����_____ �������ӳɷ�Ӧ�Ļ�ѧ����ʽΪ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com