��2013?����һģ������ԭ�Ϻ췯�ƣ��ظ����ƣ�Na
2Cr
20
7-2H
20����Ҫ���Ը�������Ҫ�ɷ�ΪFeO-Cr
2O
3��������A1
20
3��Si0
2�����ʣ�Ϊ��Ҫԭ������������Ҫ����������ͼ1��
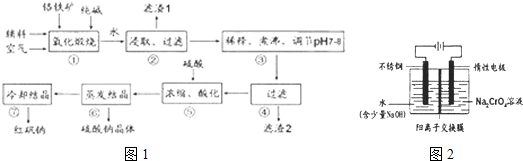
���������Ҫ��Ӧ�Ļ�ѧ����ʽΪ��Fe0?Cr
2O
3+8Na
2C0
3+70
2=8Na
2Cr0
4+2Fe
2O
3+8C0
2��I�����з�Ӧ���ڻ�תҤ�н��У���Ӧʱ�費�Ͻ��裬��������
����Ӧ��ĽӴ�������ӿ췴Ӧ����
����Ӧ��ĽӴ�������ӿ췴Ӧ����
��
��2������A1
20
3��SiO
2�ڢ���ת���Ļ�ѧ��Ӧ����ʽΪ
Al
2O
3+Na
2CO
32NaAlO
2+CO
2����SiO
2+Na
2CO
3Na
2SiO
3+CO
2��
Al
2O
3+Na
2CO
32NaAlO
2+CO
2����SiO
2+Na
2CO
3Na
2SiO
3+CO
2��
��3���û�ѧƽ���ƶ�ԭ��˵��������е�������
ˮ�����ȣ���дٽ�AlO2-+2H2O?Al��OH��3+OH-��SiO32-+H2O?HSiO3-+OH-ˮ��ƽ�������ƶ������������������������
ˮ�����ȣ���дٽ�AlO2-+2H2O?Al��OH��3+OH-��SiO32-+H2O?HSiO3-+OH-ˮ��ƽ�������ƶ������������������������
�������ӷ���ʽ�������˵������������pH���Ͳ�����Ӱ����
H+��ˮ�����ɵ��������������ܽ⣬����Al3+����Na2CrO4����
H+��ˮ�����ɵ��������������ܽ⣬����Al3+����Na2CrO4����
��4�������ữ��ʹCr0
42һת��ΪCr
20
72һ��д���÷�Ӧ�����ӷ���ʽ��
2CrO
42-+2H
+
Cr
2O
72-+H
2O
2CrO
42-+2H
+
Cr
2O
72-+H
2O
��5����ҵ�ϻ����õ�ⷨ�Ʊ��ظ����ƣ���װ��ʾ��ͼ��ͼ2��
���������ĵ缫��ӦʽΪ��
������4H2O+4e-�T4OH-+2H2����2H2O+2e-�T2OH-+H2��
��4H++4e�T2H2����2H++2e�TH2��
������4H2O+4e-�T4OH-+2H2����2H2O+2e-�T2OH-+H2��
��4H++4e�T2H2����2H++2e�TH2��
���������ĵ缫��ӦʽΪ��
������2H2O-4e-�TO2��+4H+��4OH--4e-�TO2��+2H2O
������2H2O-4e-�TO2��+4H+��4OH--4e-�TO2��+2H2O
��


 Cr2O72-+H2O
Cr2O72-+H2O Cr2O72-+H2O
Cr2O72-+H2O Cr2O72-+H2O���ʴ�Ϊ��2CrO42-+2H+
Cr2O72-+H2O���ʴ�Ϊ��2CrO42-+2H+ Cr2O72-+H2O��
Cr2O72-+H2O�� Cr2O72-+H2O����5�������ӷŵ�˳��ĽǶȷ�����
Cr2O72-+H2O����5�������ӷŵ�˳��ĽǶȷ�����


 ��2013?����һģ������ͼ��ʾװ�ý�������ʵ�飬����һ��ʱ���ʵ������Ԥ�������һ�µ��ǣ�������
��2013?����һģ������ͼ��ʾװ�ý�������ʵ�飬����һ��ʱ���ʵ������Ԥ�������һ�µ��ǣ�������