
 NH4Al��SO4��2��ʳƷ�ӹ�����Ϊ��ݵ�ʳƷ���Ӽ������ڱ���ʳƷ�У�NH4HSO4�ڷ����Լ���ҽҩ�����ӹ�ҵ����;�㷺����ش��������⣺
NH4Al��SO4��2��ʳƷ�ӹ�����Ϊ��ݵ�ʳƷ���Ӽ������ڱ���ʳƷ�У�NH4HSO4�ڷ����Լ���ҽҩ�����ӹ�ҵ����;�㷺����ش��������⣺���� ��1��Al3+ˮ�����ɵ�Al��OH��3���壬���������ԣ�
��2��NH4Al��SO4��2��Al3+ˮ�����������NH4+ˮ�⣬HSO4-�����H+ͬ������NH4+ˮ�⣻
��3����NH4Al��SO4��2ˮ�⣬��Һ�����ԣ������¶���ˮ��̶�����
�ڸ��ݵ���غ㶨�ɽ��⣮
��� �⣺��1��Al3+ˮ�����ɵ�Al��OH��3���壬���������ԣ����ӷ���ʽ��Al3++3H2O�TAl��OH��3����+3H+���ʴ�Ϊ��Al3+ˮ�����ɵ�Al��OH��3���壬���������ԣ���Al3++3H2O�TAl��OH��3����+3H+��Al��OH��3������������ʹ������Ӷ�����ˮ��
��2��NH4Al��SO4��2��NH4HSO4�е�NH4+������ˮ�⣬����NH4Al��SO4��2��Al3+ˮ�����������NH4+ˮ�⣬HSO4-�����H+ͬ������NH4+ˮ�⣬��ΪHSO4-�������ɵ�H+Ũ�ȱ�Al3+ˮ�����ɵ�H+Ũ�ȴ�����NH4HSO4��NH4+ˮ��̶ȱ�NH4Al��SO4��2�е�С���ʴ�Ϊ��С�ڣ�
��3����NH4Al��SO4��2ˮ�⣬��Һ�����ԣ������¶���ˮ��̶�����pH��С�����ϵ�����Ϊ��
�ʴ�Ϊ����NH4Al��SO4��2ˮ�⣬��Һ�����ԣ������¶ȣ���ˮ��̶�����pH��С��
�ڸ��ݵ���غ㣬�������2c��SO42-��-c��NH4+��-3c��Al3+��=c��H+��-c��OH-��=10-3 mol•L-1[c��OH-��̫С���ɺ���]���ʴ�Ϊ��10-3 mol•L-1��
���� ���⿼�������ˮ�������Ũ�ȴ�С�ıȽϣ���Ŀ�ѶȽϴ�ע��������Һ������Ũ�ȴ�С�͵���غ��غ�ķ���Ӧ�ã�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ������ | Na2CO3 | NaHCO3 | NaCl |
| ������kg�� | 814.8 | 400.3 | 97.3 |
| ������ | Na2CO3 | NaHCO3 | NaCl |
| ������kg�� | 137.7 | 428.8 | 97.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | 3��2 | B�� | 4��3 | C�� | 4��1 | D�� | 2��l |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��ѧ�� | P-P | P-O | O=O | P=O |
| ����/kJ•mol-1 | 197 | 360 | 499 | X |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ij��ɫ��Һ��CO32-NO3- Al3+ Ba2+ | |
| B�� | ʹ��̪������Һ��Na+ Cl- SO42-AlO2- | |
| C�� | ����Al�ܷų�H2����Һ�� NH4+��Cl-��Na+��SO42- | |
| D�� | ������Һ�У�NO3-��SO32-��Na+��Fe3+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���Ͽ� NO��2006-KI-001 ���⣺��������Ļ�ԭ�� �ٿα��ϣ����������ˮ��Һ�еμ�BaCl2��Һ���ٵμ�0.5mL3%�Ĺ���������Һ����Ƭ�̺�μ�ϡ���ᣬ�а�ɫ�������� �ڡ�����ѧ���²ᣬP658���������ǽ�ǿ�Ļ�ԭ�������Խ�Cl2��I2��MnO4-��ԭΪCl-��I-��Mn2+���磺 H2SO3+I2+H2O�TH2SO4+2HI |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
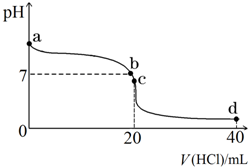 �����£�����0.1mol•L-1�������20mL 0.1mol•L-1��ˮ�У���ҺpH�������������ı仯������ͼ��ʾ��
�����£�����0.1mol•L-1�������20mL 0.1mol•L-1��ˮ�У���ҺpH�������������ı仯������ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����Ͷ���������ʹƷ����Һ��ɫ | |
| B�� | ������������ϩ����ʹ��ˮ����ɫ | |
| C�� | �������ƺ���ϩ����ʹ���Ը��������Һ��ɫ | |
| D�� | ����̿������������Һ����ʹ��������������ɫ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com