

��1��A���Լ�Ϊ______________��
��2��ʵ��ǰ���Ƚ���þ�Ͻ���ϡ���н���Ƭ�̣���Ŀ����____________________________��
��3����������ԣ���ҩƷ��ˮװ��������У����Ӻ�װ�ú�����еIJ������У��ټ�¼C��Һ��λ�ã��ڽ�B��ʣ�������ˡ�ϴ�ӡ�������أ��۴�B�в���������������ָ������º�¼C��Һ��λ�ã�����A��B�еμ������Լ����ݼ�������ԡ�����������˳����___________��������ţ���¼C��Һ��λ��ʱ��������ƽ���⣬��Ӧ_______________��
��4��B�з�����Ӧ�Ļ�ѧ����ʽΪ_________________________________________________��
��5����ʵ������þ�Ͻ������Ϊa g������������Ϊb mL���ѻ���Ϊ��״������B��ʣ����������Ϊc g�����������ԭ������Ϊ_______________��
��6��ʵ������У���δϴ�ӹ������õIJ����������������������________���ƫ����ƫС����������Ӱ�족����
(1)NaOH��Һ
(2)��ȥ��þ�Ͻ���������Ĥ
(3)�ݢ٢ܢۢ� ʹD��C��Һ����ƽ
(4)2Al+2NaOH+2H2O![]() 2NaAlO2+3H2����2Al+2OH-+2H2O
2NaAlO2+3H2����2Al+2OH-+2H2O![]() 2
2![]() +3H2��
+3H2��
(5)![]()
(6)ƫС
��������1���ɸ���Mg��Al���ʵIJ�ͬ��ѡ���Լ���Al����NaOH��Һ��Ӧ����Mg�����ԣ���ѡ��NaOH��Һ��
��2����þ���Ͻ���ϡ���н���Ƭ�̣�Ŀ����Ϊ�˳�ȥ�Ͻ���������Ĥ��
��3����ʵ��ǰӦ����װ�������ԣ�����¼C��Һ��λ�ã�����A��B.�м�������NaOH��Һ��ֱ����Ӧ���������һָ������£��ٴμ�¼C��Һ��λ�ã����ʣ�����ϴ�ӡ�������أ���˳��Ϊ�ݢ٢ܢۢڣ�����ʱ��������ƽ���⣬��Ҫע��C��D������Һ����ƽ����Ҫ����Һ��Ӱ�������
��4��Al��NaOH��Һ������Ӧ��2Al+2NaOH+2H2O![]() 2NaAlO2+3H2��
2NaAlO2+3H2��
��2Al+2OH-+2H2O![]() 2
2![]() +3H2��
+3H2��
��5��m��Al��=��a-c��g��n��Al��=![]() n��H2��=
n��H2��=![]() ��
��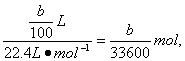
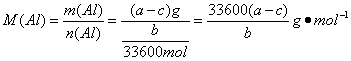 Al�����ԭ������Ϊ
Al�����ԭ������Ϊ![]() ��
��
��6����δϴ�ӹ������ò�������cֵƫ��m��Al��=��a-c�� gƫС����Al�����ԭ������ֵƫС��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
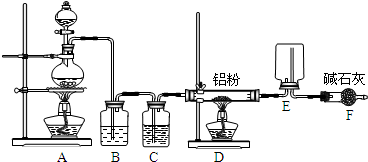
��8�֣�ijѧϰС������ͼװ�òⶨ��þ�Ͻ������������������������ԭ������.
����ʾ��þ���������ᷴӦ�������ܺͼӦ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
ʵ��ǰ���Ƚ���þ�Ͻ���ϡ���н���Ƭ�̣���Ŀ���dz�ȥ��þ�Ͻ���������Ĥ��
��1��A���Լ�Ϊ .
���NaOH��Һ����ϡ���ᡱ��
��2����������ԣ���ҩƷ��ˮװ��������У����Ӻ�װ�ú�����еIJ������У��ټ�¼
C��Һ��λ�ã��ڽ�B��ʣ�������ˣ�ϴ�ӣ�������أ��۴�B�в���������������ָ������£�����A��B�еμ������Լ����ݼ�������ԣ�����������ʹD��C��Һ����ƽ��
����������˳���� ��������ţ�
��3��B�з�����Ӧ�����ӷ���ʽΪ .
��4��ʵ������У���δϴ�ӹ������õIJ���������������������� .
���ƫ����ƫС����������Ӱ�족��
��5����ʵ������þ�Ͻ������Ϊa g,����������Ϊb ml���ѻ���Ϊ��״������B��ʣ����������Ϊc g�����������ԭ������Ϊ .���ú�a��b��c�Ĵ���ʽ��ʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012������ʡ����һ�и�����ѧ�ڵڶ���������⻯ѧ�Ծ� ���ͣ�ʵ����
��12�֣�ijѧϰС������ͼװ�òⶨ��þ�Ͻ������������������������ԭ��������
��1��A���Լ�Ϊ______________��
��2��ʵ��ǰ���Ƚ���þ�Ͻ���ϡ���н���Ƭ�̣���Ŀ����_________________________��
��3����������ԣ���ҩƷ��ˮװ��������У����Ӻ�װ�ú�����еIJ������У��ټ�¼C��Һ��λ�ã��ڽ�B��ʣ�������ˡ�ϴ�ӡ�������أ��۴�B�в���������������ָ������º�¼C��Һ��λ�ã�����A��B�еμ������Լ����ݼ�������ԡ�����������˳����___________��������ţ���¼C��Һ��λ��ʱ��������ƽ���⣬��Ӧ_______________��
��4��B�з�����Ӧ�Ļ�ѧ����ʽΪ____________________________________________��
��5����ʵ������þ�Ͻ������Ϊa g������������Ϊb mL���ѻ���Ϊ��״������B��ʣ����������Ϊc g�����������ԭ������Ϊ_______________��
��6��ʵ������У���δϴ�ӹ������õIJ����������������������________���ƫ����ƫС����������Ӱ�족����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012������ʡ��ԭ���и�һ��ѧ�ڵ�һ�ο��Ի�ѧ�Ծ� ���ͣ�ʵ����
��8�֣�ijѧϰС������ͼװ�òⶨ��þ�Ͻ������������������������ԭ������.
����ʾ��þ���������ᷴӦ�������ܺͼӦ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
ʵ��ǰ���Ƚ���þ�Ͻ���ϡ���н���Ƭ�̣���Ŀ���dz�ȥ��þ�Ͻ���������Ĥ��
��1��A���Լ�Ϊ .
���NaOH��Һ����ϡ���ᡱ��
��2����������ԣ���ҩƷ��ˮװ��������У����Ӻ�װ�ú�����еIJ������У��ټ�¼
C��Һ��λ�ã��ڽ�B��ʣ�������ˣ�ϴ�ӣ�������أ��۴�B�в���������������ָ������£�����A��B�еμ������Լ����ݼ�������ԣ�����������ʹD��C��Һ����ƽ��
����������˳���� ��������ţ�
��3��B�з�����Ӧ�����ӷ���ʽΪ .
��4��ʵ������У���δϴ�ӹ������õIJ���������������������� .
���ƫ����ƫС����������Ӱ�족��
��5����ʵ������þ�Ͻ������Ϊa g,����������Ϊb ml���ѻ���Ϊ��״������B��ʣ����������Ϊc g�����������ԭ������Ϊ .���ú�a��b��c�Ĵ���ʽ��ʾ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com