�����£�N
2H
4���͵����ᶼ�ǵ�Ԫ�ص���Ҫ�⻯�
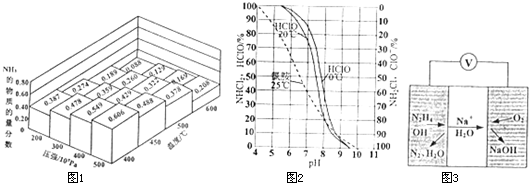
��1�������������쵪�ʡ�����ȣ�
�ٺϳɰ���ҵ�У����������йط�Ӧ���Ȼ�ѧ����ʽ���£�
C��s��+H
2O��g��=CO��g��+H
2��g����H
1=+131.4kJ?mol
-1C��s��+2H
2O��g��=CO
2��g��+2H
2��G����H
2=+90.2kJ?mol
-1CO��g��+H
2O��g��=CO
2��g��+H
2��g����H
3���H
3=
��
����һ�ܱ������У��������ʵ���֮��Ϊ1��3��N
2��H
2���ڲ�ͬ�¶ȡ�ѹǿ�²��ƽ����ϵ��NH
3�����ʵ���������ͼ1��ʾ�����¶�Ϊ400�桢ѹǿΪ500��10
5 Paʱ��H
2��ƽ��ת������ӽ�
������ţ���
A.89% B.75%
C.49% D.34%
��ʵ�������У��ϳɰ����¶�һ�������400��500�棬ѡ����¶ȷ�Χ��������
��
������ˮ�Ȼ�����ʱ��ˮ�к��а��������Ȱ���NH
2Cl��NHCl
2�ȣ����Ȱ�ˮ����ͷų�HClO�������õ�����Ч����ͼ2������ˮ�Ȼ�����ʱ�йسɷֵĺ�����pH�Ĺ�ϵ������˵����ȷ����
������ţ���
A��HCIO��20��ĵ���̶�һ������0��ĵ���̶�
B��pHԽ������Ч��Խ��
C����NH
2Cl����ʱ��ƽ��NH
2Cl+H
2O?NH
3+HClO�����ƶ�
��2���¿����ڻ��ȼ�ϡ���ҩԭ�ϵȣ�
�ٴ�������������İ���Ӧ�����Ʊ��£��÷�Ӧ�Ļ�ѧ����ʽΪ
��
��һ����ȼ�ϵ�صĹ���ԭ����ͼ3��ʾ���õ�ع���ʱ�����ĵ缫��ӦʽΪ
��
�����������ᣨHNO
2����Ӧ�����ɵ����ᣮ8.6g��������ȫ�ֽ�ɷų�6.72L��������״���£����������ķ���ʽΪ
��





 ��2013?������һģ���£�N2H4���Ͱ��ǵ������ֳ���������ڿ�ѧ�������������й㷺Ӧ�ã��밴Ҫ��ش��������⣺
��2013?������һģ���£�N2H4���Ͱ��ǵ������ֳ���������ڿ�ѧ�������������й㷺Ӧ�ã��밴Ҫ��ش��������⣺

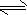 N2H5++OH-
N2H5++OH-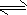 N2H62++OH-
N2H62++OH-  N2H4+H3O+
N2H4+H3O+