
Ŀǰ���������Ѿ�ʹ���Ҵ����͡������Ҵ������ɺ����۵�ũ��Ʒ�����ȷ��͡�������á�
(1)������л�ѧ����ʽ��
���ۣ�ˮ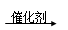 �����ǣ�___________________________________
�����ǣ�___________________________________
������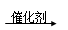 �Ҵ���____________________________________��
�Ҵ���____________________________________��
(2)��֪��ʢ����������Һ��ij�����м����ĸ����������Һ������180 g������ȫ�������ꡣ���˹����в���������CO2�����õ��ܵ��������ij���ʯ��ˮ�У���������ɫ����400 g���˹����в����ľƾ�����Ϊ________ g��
(3)�����Ҵ����ͳ�Ϊ����ȼ�ϵ�ԭ����________________________________________
(1)(C6H10O5)n(����)��nH2O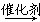 nC6H12O6(������)
nC6H12O6(������)
C6H12O6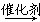 2C2H5OH��2CO2��
2C2H5OH��2CO2��
(2)46
(3)�Ҵ���������Ч�ؽ�������β�������Ĵ�����Ⱦ��
����������1����������ˮ�ⷽ��ʽ����д��
��2��n(������)��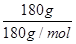 ��1 mol
��1 mol
n(CO2)��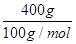 ��4 mol
��4 mol
��1 mol����������ֻ����1 mol C2H5OH
������1mol��46g/mol��46g
��Ӧ�ķ���ʽ��C6H12O6��3O2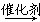 C2H5OH��4CO2����3H2O
C2H5OH��4CO2����3H2O
��3�������Ҵ���ȼ�ղ�����ˮ��CO2�������Ҵ���������Ч�ؽ�������β�������Ĵ�����Ⱦ��


 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꼪��ʡ����һ�и߶�10���¿���ѧ�Ծ����������� ���ͣ������
Ŀǰ���������Ѿ�ʹ���Ҵ����͡������Ҵ������ɺ����۵�ũ��Ʒ�����ȷ��͡�������á�
(1)������л�ѧ����ʽ��
���ۣ�ˮ �����ǣ�___________________________________
�����ǣ�___________________________________
������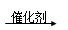 �Ҵ���____________________________________��
�Ҵ���____________________________________��
(2)��֪��ʢ����������Һ��ij�����м����ĸ����������Һ������180 g������ȫ�������ꡣ���˹����в���������CO2�����õ��ܵ��������ij���ʯ��ˮ�У���������ɫ����400 g���˹����в����ľƾ�����Ϊ________ g��
(3)�����Ҵ����ͳ�Ϊ����ȼ�ϵ�ԭ����________________________________________
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com