
ij�о��ԡ�ѧϰС����������װ��̽�������백��֮��ķ�Ӧ�� ����A��F�ֱ�Ϊ�����������ķ���װ�ã�CΪ�����백����Ӧ��װ�á�

��ش��������⣺
��1����μ��װ��A�������ԣ�
��
��2��װ��F�з�����Ӧ�����ӷ���ʽ�� ��
��3��װ��A��ƿ�ڵĹ������ѡ�� ������ţ���
a����ʯ�� b����ʯ�� c���������� d������������ e���ռ�
��4�����߿�B��D��E��Ӧ���ӱ�Ҫ�����徻��װ�ã������ͼ�ġ���ѡװ�á���ѡ���Ҫ��װ�ã�����������ո��У�
B ��D ��E ��
��5�������Ͱ�����Ӧ���������Ȼ�泥�ʵ���й۲쵽װ��C�ڳ���Ũ��İ��̣�����������������ɰ�ɫ���壬�����ʵ�鷽��ȷ���ù������Ȼ�泥���д���пհף����Բ���������
�ٲ����� ������ͽ��ۣ� ��
�ڲ����� ������ͽ��ۣ� ��
�۲����� ������ͽ��ۣ� ��
��6����װ�û��в���֮��������������ǸĽ��� ��
��1������һ��������Ƥ������ֹˮ�м�ס��Ƥ���ܣ�����K�ӷ�Һ©������ƿ�з�ˮ��һ��ʱ���Һ©���ڵ�ˮ���ٵ��£�˵��Aװ�ò�©����
��������������Ƥ�����رջ���K��������ĩ������ʢˮ���ձ��У�����ƿ�����ܿ�������ð����ֹͣ���ȣ�����ĩ���γ�һ��ˮ����
��2��MnO2+4H++2C1![]() Mn2++C12��+2H2O
Mn2++C12��+2H2O
��3��a��b��e
��4��I����
��5����ȡ��ɫ������ŨNaOH��Һ���ȣ�������������ʹʪ��ĺ�ɫʯ����ֽ������˵������NH4+
����ȡ��ɫ��������ˮ���μ��������ữ��AgNO3��Һ��������ɫ������˵������Cl�D
��6��������G��װ��NaOH��Һ��ϴ��ƿ��������β��ϴ�Ӻ����ſա�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��㶫ʡ������ֱ����У�����ڶ���������2�£����ۻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
ij��ȤС����Ʋ�����������ʵ������ȡ��ˮ��������������ˮ���ʵ�顣
��1��ʵ������������װ���Ʊ���ˮ���밴������������������ķ�����������������
H��������_____________����ӿڴ��ķ��ţ������ƿ���е��Լ�Ϊ��������������������

��2��д����ʵ������ȡCl2�Ļ�ѧ����ʽΪ��___________________________________
��3��ij��ȤС�����ʵ���ȥ�Ȼ����к���Fe3+��SO42-��Br-���������ӣ��������£�
A���ܽ⣬��������Һ�м����Լ���Fe3+��SO42-������ȫ����У�
B�����ˣ�����Һ�м����������pH��
C������_____����Br-�����������ա���ش����⣺
��3��������a�У����μ�����Լ���NH3.H2O��_________��_________��
������c�м�����Լ���__________________��
��4��ij�о���ѧϰС����KSCN����FeSO4��Һ�е�Fe2+ʱ���������ŨHNO3����Һ����ɫ��졣���ǽ���ɫ��Һ����һ��������Һ�ɺ�ɫͻȻ��Ϊ��ɫ������������ɫ���塣����һ��������ͬѧ�ǽ���������̽����
�۲������ϣݣ�
(SCN)2��Ϊ��±�أ�������±�ص������ƣ�(SCN)2��Cl2��Br2��I2�������ʵ�������ǿ��Ϊ��Cl2��Br2��(SCN)2��I2��
���������ݣ�
����ͬѧ������FeSO4��HNO3���ã���ͬѧ������______��HNO3���á�
��ʵ����֤�ݣ�
����Ҿ������Է�������Ϊ��ͬѧ�ļ��費������������________________________��
��������ͬѧ�ļ��裬���������ʵ�鷽��������֤����ŨHNO3����μ���KSCN��Һ��ʵ�鿪ʼʱ����������һ��ʱ�����Һ�������ɫ����ɫ��ͻȻ���ҷ�Ӧ�����������ݣ��ų�����ɫ���塣������������ͨ�������Ba(OH)2��Һ���������ǣ���ʣ��һ�����壨�ǿ����е���Ҫ�ɷ�֮һ������Ӧ�����Һ�м���BaCl2��Һ������ɫ������
��Ӧ�в����������ǣ�_______________________________
��ʵ����ۣݣ���ͬѧ������ȷ��
��ʵ�鷴˼�ݣ�
����ʵ���֪����SCN-��Ӽ���Fe2+ʱ�����������������HNO3�����ѡ����ˮ�ȣ�ͨ����ʵ��̽����֪������������������Ҫ_______��������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���˱�ģ�� ���ͣ��ʴ���
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ѧ�Һ���?Ī��ɣһ���ڻ�ѧԪ�ط���ʷ���������ش��ס�Ī��ɣ���������ѧ�о���������ȼ����������������ʯ����Ī��ɣ���������������������¼��Ȼ�ԭ���Ƶ�����ȼ����������ϸ�Ľ��������ڿ�����ײ�������Ȼ�ȼ�գ���
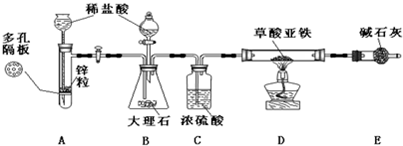
![]() ij�о���ѧϰС����ģ��Ī��ɣ��չ��ʵ�����Ʊ���ȼ���о�������û���ִ�����������������ϵ�֪����ͨ���ڸ������������¼��Ȳ���������FeC2O4���Ƶ����������� FeC2O4
ij�о���ѧϰС����ģ��Ī��ɣ��չ��ʵ�����Ʊ���ȼ���о�������û���ִ�����������������ϵ�֪����ͨ���ڸ������������¼��Ȳ���������FeC2O4���Ƶ����������� FeC2O4![]() FeO��CO����CO2��
FeO��CO����CO2��
�ɴˣ�С�������һ�����Ʊ������������̺��Ʊ���ȼ����װ�ã�����̨�ȹ̶�����װ����ͼ����ȥδ������
![]()

��������װ�õ�ԭ���ش��������⣺
��1��д�������������Ʊ���ȼ���Ļ�ѧ����ʽ��
��2��������װ�ú�����Ҫ�����ǣ�
���������
��3��ʵ����Bװ�õ������ǣ� ��
��4�����еڶ����Ʊ���ȼ��ʵ�鲽�����ȷ˳���ǣ�
�ٵ�ȼD���ƾ��ƣ� �ڹر�A��B֮��Ļ������أ���Ϩ��ƾ��ƣ�����D�IJ�����ͨ����㹻ʱ��������ų�ԭ�������壬�ݼ���ͨ������D�IJ����ڹ�����ȴ�������������ߴ�A��B֮��Ļ�������
��5������װ����û�����Ե����⣿ ������,�ش���θĽ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009���Ĵ�ʡ�˱��и߿���ѧ�����Ծ��������棩 ���ͣ������
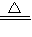 FeO+CO��+CO2��
FeO+CO��+CO2��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com