
 ��1����ijҩƷ����ԼΪ32g����������ƽȷ�������������á���ʾ�����̷������룬�á���ʾ������ȡ�£������б���Ŀո��ڣ��á��͡���ʾ��Ӧ����ķ��ϻ�ȡ�£�
��1����ijҩƷ����ԼΪ32g����������ƽȷ�������������á���ʾ�����̷������룬�á���ʾ������ȡ�£������б���Ŀո��ڣ��á��͡���ʾ��Ӧ����ķ��ϻ�ȡ�£�| 50g | 20g | 20g | 10g | 5g |
���� ���� |
�� �� |
���� ���� |
�� �� |
���� ���� |
| n |
| V |
| 50g | 20g | 20g | 10g | 5g |
| ���� | �� | ���� | �� | ���� |


 ������״Ԫ���Ծ�ϵ�д�
������״Ԫ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ҫ����գ�
��1����ijҩƷ����ԼΪ32g����������ƽȷ�������������á���ʾ�����̷������룬�á���ʾ������ȡ�£������б���Ŀո��ڣ��á��͡���ʾ��Ӧ����ķ��ϻ�ȡ�¡�
| 50g | 20g | 20g | 10g | 5g |
| ���� | �� |
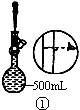
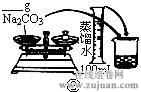
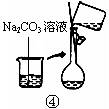
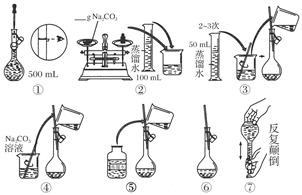
��2������500mL 0.1mol��L -1 Na2CO3��Һ����ͼ��������Ӧ����д������Ϊ ��ʵ��ʱ��ͼ��ʾ�������Ⱥ�˳��Ϊ �����ţ���

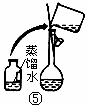

��3��������һ���������ʵ���Ũ����Һʱ���á�ƫ�ߡ�ƫ�͡���Ӱ�족��ʾ���в�����������ҺŨ�ȵ�Ӱ�졣
�ٶ���ʱ���ӣ���������Һ��Ũ�� ��
������ƿϴ�Ӻ�δ�����������Һ��Ũ�� ��
�۶���ҡ�Ⱥ���������Һ��������������Һ��Ũ�� ��
��4������̼���ƹ��壬������ˮ�У����� ����Ӧ�ķ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
[s1] ��1����ijҩƷ����ԼΪ32��0 g,��������ƽȷ�������������á���ʾ�����̷������룬�á���ʾ������ȡ�£������б���Ŀո��ڣ��á��͡���ʾ��Ӧ����ķ��ϻ�ȡ�¡�
| 50 g | 20 g | 20 g | 10 g | 5 g |
|
|
|
|
|
|
 |
��3��������һ�����ʵ���Ũ�ȵ���Һʱ���á�ƫ�ߡ�ƫ�͡���Ӱ�족��ʾ���в�����������ҺŨ�ȵ�Ӱ�졣
������Ͳ��ȡҺ̬���ʣ�����ʱ��������Ͳ����������Һ��Ũ�� ��
�ڽ���ȡҺ̬���ʵ���Ͳ��ˮϴ�ӣ�ϴ��Һ��������ƿ����������Һ��Ũ�� ��
�۶���ҡ�Ⱥ���������Һ����������������Һ��Ũ�� ��
[s1]12��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�������ѧ�ڻ�ѧһ�ָ�ϰ����ѧ�����е��������硷ר���ۺϲ��ԣ��ս̰棩 ���ͣ�ʵ����
(1)��ijҩƷ����ԼΪ32 g����������ƽȷ�������������á�������ʾ�����̷������룬�á�������ʾ������ȡ�£������б����ڣ��á������͡�������ʾ��Ӧ����ķ��ϻ�ȡ�¡�
| 50 g | 20 g | 20 g | 10 g | 5 g |
| | | | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010����������������ٵݡ�ϵ�У�21�� ���ͣ������
[s1] ��1����ijҩƷ����ԼΪ32��0 g,��������ƽȷ�������������á���ʾ�����̷������룬�á���ʾ������ȡ�£������б���Ŀո��ڣ��á��͡���ʾ��Ӧ����ķ��ϻ�ȡ�¡�
|
50 g |
20 g |
20 g |
10 g |
5 g |
|
|
|
|
|
|
 |
��3��������һ�����ʵ���Ũ�ȵ���Һʱ���á�ƫ�ߡ�ƫ�͡���Ӱ�족��ʾ���в�����������ҺŨ�ȵ�Ӱ�졣
������Ͳ��ȡҺ̬���ʣ�����ʱ��������Ͳ����������Һ��Ũ�� ��
�ڽ���ȡҺ̬���ʵ���Ͳ��ˮϴ�ӣ�ϴ��Һ��������ƿ����������Һ��Ũ�� ��
�۶���ҡ�Ⱥ���������Һ����������������Һ��Ũ�� ��
[s1]12��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com