
�û�ѧ��Ӧ�����뻯ѧƽ�⼰ƽ���ƶ���ԭ�����Է�������ƽ�⣬�磺(����ƽ��)CH3COOH![]() CH3COO����H+��(�ܽ�ƽ��)Ca(OH)2(s)
CH3COO����H+��(�ܽ�ƽ��)Ca(OH)2(s)![]() Ca2+(aq)��2OH��(aq)��(���ƽ��)Fe3+��SCN����[Fe(SCN)]2+�ȣ�������ˮ������ˮ�д����ܽ�ƽ�⣬�䱥����Һ(�ﵽ�ܽ�ƽ��ʱ����Һ)�и����ӵ�Ũ����ѭһ���Ķ�����ϵ������AgCl��ˮ�д���ƽ�⣺AgCl
Ca2+(aq)��2OH��(aq)��(���ƽ��)Fe3+��SCN����[Fe(SCN)]2+�ȣ�������ˮ������ˮ�д����ܽ�ƽ�⣬�䱥����Һ(�ﵽ�ܽ�ƽ��ʱ����Һ)�и����ӵ�Ũ����ѭһ���Ķ�����ϵ������AgCl��ˮ�д���ƽ�⣺AgCl![]() Ag+��Cl������һ���¶��£�������Һ��Ag+�����ʵ���Ũ�Ⱥ�Cl�������ʵ���Ũ�ȵij˻�Ϊһ����������Ksp��ʾKsp��c(Ag+)��c(Cl��)����֪̼��ƺ�����������ˮ�д��������ܽ�ƽ�⣺Ca(OH)2(s)
Ag+��Cl������һ���¶��£�������Һ��Ag+�����ʵ���Ũ�Ⱥ�Cl�������ʵ���Ũ�ȵij˻�Ϊһ����������Ksp��ʾKsp��c(Ag+)��c(Cl��)����֪̼��ƺ�����������ˮ�д��������ܽ�ƽ�⣺Ca(OH)2(s)![]() Ca2+(aq)��2OH��(aq)��2CaCO3(s)
Ca2+(aq)��2OH��(aq)��2CaCO3(s)![]()
![]() ���ڻ������糧ȼ��ú�ķ�������������SO2��O2��N2��CO2�ȣ�Ϊ�˳�ȥ�к�����SO2�����Ϊ���������÷�ĩ״��̼��ƻ���ʯ�ҵ�����Һϴ�ӷ�������Ӧ����Ϊʯ�࣮
���ڻ������糧ȼ��ú�ķ�������������SO2��O2��N2��CO2�ȣ�Ϊ�˳�ȥ�к�����SO2�����Ϊ���������÷�ĩ״��̼��ƻ���ʯ�ҵ�����Һϴ�ӷ�������Ӧ����Ϊʯ�࣮
˼���⣺
(1)д������������Ӧ�Ļ�ѧ����ʽ��
(2)��˵������ʯ�ҵ�����Һ�����ó���ʯ��ˮ�����ɣ�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 pD��g��+qE��s������H��0��m��n��p��qΪ��������ȣ���
pD��g��+qE��s������H��0��m��n��p��qΪ��������ȣ���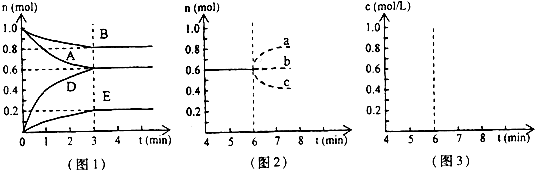


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
CH3COOH![]() CH3COO��+H+������ƽ�⣩
CH3COO��+H+������ƽ�⣩
Ca(OH)2(s) ![]() Ca2��+2OH�����ܽ�ƽ�⣩
Ca2��+2OH�����ܽ�ƽ�⣩
*Fe3��+SCN��![]() ��Fe(SCN)��2�������ƽ�⣩��
��Fe(SCN)��2�������ƽ�⣩��
������ˮ������ˮ�д����ܽ�ƽ�⣬�䱥����Һ���ﵽ�ܽ�ƽ��ʱ����Һ���и����ӵ�Ũ����ѭһ���Ķ�����ϵ������AgCl��ˮ�д���ƽ�⣺AgCl![]() Ag++Cl������һ���¶��£�������Һ��Ag+�����ʵ���Ũ�Ⱥ�Cl�������ʵ���Ũ�ȵij˻�Ϊһ����������Ksp��ʾ��Ksp=c(Ag+)��c(Cl��)����֪̼��ƺ�����������ˮ�д��������ܽ�ƽ�⣺Ca(OH)2(s)
Ag++Cl������һ���¶��£�������Һ��Ag+�����ʵ���Ũ�Ⱥ�Cl�������ʵ���Ũ�ȵij˻�Ϊһ����������Ksp��ʾ��Ksp=c(Ag+)��c(Cl��)����֪̼��ƺ�����������ˮ�д��������ܽ�ƽ�⣺Ca(OH)2(s)![]() Ca2��+2OH����CaCO3(s)
Ca2��+2OH����CaCO3(s)![]() Ca2��+CO
Ca2��+CO![]() ���ڻ������糧ȼ��ú�ķ�������������SO2��O2��N2��CO2�ȡ�Ϊ�˳�ȥ�к�����SO2�����Ϊ���������÷�ĩ״��̼��ƻ���ʯ�ҵ�����Һϴ�ӷ�������Ӧ����Ϊʯ�ࡣ
���ڻ������糧ȼ��ú�ķ�������������SO2��O2��N2��CO2�ȡ�Ϊ�˳�ȥ�к�����SO2�����Ϊ���������÷�ĩ״��̼��ƻ���ʯ�ҵ�����Һϴ�ӷ�������Ӧ����Ϊʯ�ࡣ
˼������1��д������������Ӧ�Ļ�ѧ����ʽ��
��2����˵������ʯ�ҵ�����Һ�����ó���ʯ��ˮ�����ɡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��ѧ��Ӧ�����뻯ѧƽ�����ճ������ũҵ�����Ϳ�ѧ�о��о�����Ҫ�����壬��
��ѧʵ�����漰�ĵ���ƽ��Ҳ���ڻ�ѧƽ�⡣��ش��������⣺
��1��ij�о���ѧϰС��������Ϸ��ֽ���������AҲ�ܴ�����صķֽ⣬��A�Ͷ�
�����̵���Ѵ��¶Ⱦ�Ϊ500�����ҡ����Ƕ�A�Ͷ������̵Ĵ����ܽ�����
��������ʵ�顣ʵ��ʱ��������500 mL����Ϊ(��������Ӱ��ʵ������ؾ���
����)��
��һ ��MnO2������
| ʵ����� | KClO3����/g | MnO2����/g | ��Ӧ�¶�/�� | �������� |
| 1 | 8.00 | 2.00 | 500 | |
| 2 | 8.00 | 2.00 | 500 |
���� ��A������
| ʵ����� | KClO3����/g | A������/g | ��Ӧ�¶�/�� | �������� |
| 1 | 8.00 | 2.00 | 500 | |
| 2 | 8.00 | 2.00 | 500 |
��ش�����ʵ���еĴ�������Ӧ�� ��
��ɴ��о�������������һƪ�о����棬������������һ�о�����ı��⣺
��
��2����ˮ��һ�����ijͬѧȡ0.1mo/L�İ�ˮ����pH��ֽ����pH�����������Һ��
pHԼΪ11���Դ˵ó���ˮΪ����Ľ��ۡ���ͬѧ��pH��ֽ�ⶨ��ˮpH�ľ����
���� ��
��3��֤����ˮ������ij��÷����������֣�һ���跨֤��NH![]() ��ˮ�⣬һ���跨ʹ��ˮ
��ˮ�⣬һ���跨ʹ��ˮ
����ƽ�ⷢ���ƶ���
����һ��ȡ����NH4Cl��������ˮ������ʯ����Һ����Һ��죬�ɼ���Һ�����ԡ�
��ԭ���� ��
��������ȡ������ˮ�������̪�����ټ��� ������ɫ��dz��c(OH��)�½���˵����ˮ�ĵ���ƽ���� �ƶ���
�鿴�𰸺ͽ���>>
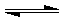
��Ŀ�����л�ѧ ��Դ��2009-2010ѧ��㶫ʡ��ͷ��������ѧ��һ���£����л�ѧ�Ծ��������棩 ���ͣ������
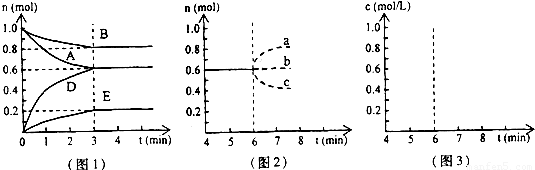
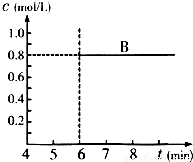
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com