
ij��Һ���ܺ���Cl����SO42����CO32-��NH4+��Fe3����Fe2����Al3����Na����ijͬѧΪ��ȷ����ɷ֣�ȡ������Һ����Ʋ����������ʵ�飺
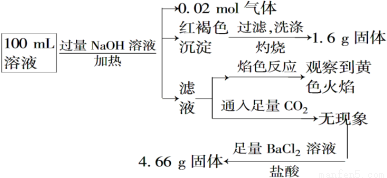
����˵����ȷ����
A��ԭ��Һ��c(Fe3��)��0.2 mol��L��1
B��Ҫȷ��ԭ��Һ���Ƿ���Fe2���������Ϊȡ����ԭ��Һ���Թ��У���KSCN��Һ���ټ���������ˮ����Һ��Ѫ��ɫ������Fe2��
C��SO42����NH4+��Na��һ�����ڣ�CO32-��Al3��һ��������
D����Һ��������4�����Ӵ��ڣ�����Cl��һ�����ڣ���c(Cl��)��0.2 mol��L��1
 ����ѧ���ʱѧ����ϵ�д�
����ѧ���ʱѧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016������ʡ������ѧ�����в��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�״����ӽ���Ĥȼ�ϵ���н��״�����ת��Ϊ���������ַ�Ӧԭ���ǣ� ��
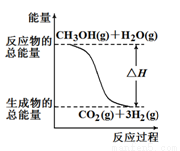
��CH3OH(g)��H2O(g)��CO2(g)��3H2(g) ��H ����49.0 kJ/mol
��CH3OH(g)��1/2O2(g)��CO2(g)��2H2(g) ��H����192.9 kJ/mol
����������Ӧ������˵����ȷ����
A����ͼ��ʾ��Ӧ���е������仯
B������֪2H2(g)��O2(g)��2H2O(g) ?��H����483.8 kJ/mol
C��1 mol CH3OH���ȼ�շų�������Ϊ192.9 kJ
D��CH3OHת���H2�Ĺ���һ��Ҫ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�꽭��ʡ�߶��ϵڶ����ʼ컯ѧ�Ծ��������棩 ���ͣ�ѡ����
ʵ�����У��й��Լ��ı��淽���������
A���ռ���Һ�����ڴ����������Լ�ƿ��
B������ᱣ��������ƿ��
C�����������Ʊ�����ú����
D��Ũ���ᱣ������ɫϸ��ƿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��ɽ��ʡ�߶���12���¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
dz��ɫ����������茶���[����Ī���Σ�(NH4)2SO4��FeSO4��6H2O]���̷���FeSO4��7H2O�����ȶ��������ڶ���������Ī���ε�һ��ʵ�����Ʒ����£�
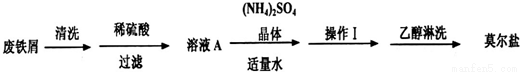
��1�������м�м���ϡ�����������м��ȫ�ܽ����ʣ������ʱ�ͽ��й��ˣ���Ŀ����_________��
��2��Ũ�Ⱦ�Ϊ0.10mol•L-1��Ī������Һ��FeSO4��Һ��c��Fe2+��ǰ��______���ߣ�������ڡ�����С�ڡ����ڡ������жϡ���
��3��0.10mol•L-1Ī������Һ������Ũ���ɴ�С��˳��Ϊ_________��
��4�������£���0.10mol•L-1��NH4��2SO4��Һ��pH=5������NH3•H2O�ĵ���ƽ�ⳣ��Kb��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ������ɽ�ϵڶ�����ѧ��һ����ĩ��ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪������������ˮ�����������л��ܼ�CCl4������װ���в�������NH3��β�����յ���
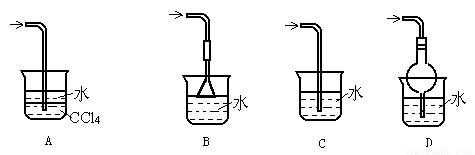
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ���㽭ʡ�߶������в��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
һ�������£���ѧ��ѧ�������ʼס���֮���������ת����ϵ������
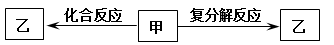
A��HCl B��NaHCO3 C��KOH D��FeCl2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016������и�����ѧ�ڵ�����¿����ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʵ���ܴﵽԤ��Ŀ�ĵ��ǣ�
A���Ӻ�I-����Һ����ȡ�⣺���������ữ��H2O2��Һ�����þƾ���ȡ
B��������Һ���Ƿ�һ������CO32-���μ�ϡ���ᣬ������������ͨ�����ʯ��ˮ
C����������ˮ������Ƿ���л�ԭ�ԣ���ˮ������Һ�м�������Cu(OH)2������
D����ȥ�����е�Ca2+��SO42-�����μ������BaCl2��Һ��Na2CO3��Һ�����˺��ټ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�����ʡ������ѧ�ڵ�����¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪ij�����£��ϳɰ���Ӧ���������£�N2��g��+3H2��g�� 2����3��g��
2����3��g��
��ʼŨ��/mol•L-1 | 1.0 | 3.0 | 0.2 |
2sĩŨ��/mol•L-1 | 0.6 | 1.8 | 1.0 |
4sĩŨ��/mol•L-1 | 0.4 | 1.2 | 1.4 |
���ð���Ũ�ȵ���������ʾ�÷�Ӧ������ʱ������˵���д������
A��2sĩ�����ķ�Ӧ����=0.4mol•��L•s��-1
B��ǰ2sʱ���ڰ�����ƽ����Ӧ����=0.4mol•��L•s��-1
C��ǰ4sʱ���ڰ�����ƽ����Ӧ����=0.3mol•��L•s��-1
D��2��4sʱ���ڰ�����ƽ����Ӧ����=0.2mol•��L•s��-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��㶫��ͷ��ɽ��ѧ�߶���12���¿���ѧ���������棩 ���ͣ�ѡ����
���н���ʵ����ʵ�ķ���ʽ�У���ȷ����
A����CH3COONa��Һ�е�����ɫ��̪����Һ���:CH3COO-+H2O CH3COOH+OH-
CH3COOH+OH-
B����Mg(OH)2��ɫ����Һ�е��뱥��FeCl3��Һ������Һ���֣�
3Mg(OH)2+2Fe3+===2Fe(OH)3+3Mg2+
C����NaHSO3��Һ�еμ���ɫʯ����Һ����Һ��죺NaHSO3=Na++H++SO32-
D����ϡH2SO4�ữ��KMnO4��Һ�е���˫��ˮ����Һ��ɫ:
2MnO4-+5H2O2+6H+===2Mn2++5O2��+8H2O
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com