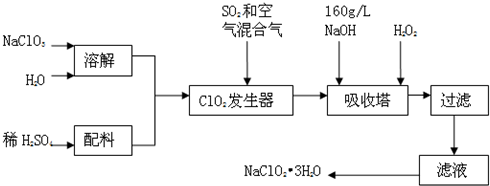
»¯ر§؟ئرذذ،×éµؤح¬ر§شع¾«ب·²âتشNa
2SO
3بـز؛µؤpHت±£¬سِµ½ءثہ§»َ£®خھ´ث£¬ثûأا½ّذذءثہن¾²µؤث¼؟¼؛ح×¼±¸£¬¾ِذؤضطذآتµر飬½ز؟ھ°آأط£®اëؤمءث½âاé؟ِ£¬²خسë½»ء÷جضآغ£®
[²éشؤ×تءد]¢ظ³£خآدآ0.1mol?L
-1µؤH
2SO
3بـز؛µؤpHش¼خھ2.1£®
¢عح¨³£اé؟ِدآ£¬H
2Sخھخقة«£¬سذ¸¯µ°³ôخ¶µؤئّجه£¬ئنث®بـز؛³ئخھاâءٍثل£®³£خآدآ0.1mol?L
-1µؤH
2Sبـز؛µؤpHش¼خھ4.5£®
[تµرé²ظ×÷]¢ظ×¼ب·³ئب،´؟¾»µؤNa
2SO
3?7H
2O¾§جه25.20g£¬إن³ة1Lث®بـز؛£¬²âµأئنpH=7.8£®
¢عزشغلغِشظ´خ×¼ب·³ئب،25.20gةدتِ¾§جه£¬¼ج¶ّ¸ô¾ّ؟صئّشع600،وزشةد¸كخآدآا؟ببضء؛مضط£¬ضتء؟خھ12.60g£®
¢غ½«¢عثùµأض®12.60g¹ججه½ّذذشھثط¶¨ذش¶¨ء؟·ضخِ£¬×é³ةسëNa
2SO
3خقزى£®½«ئنبـسعث®µأ250mLبـز؛£¬²âµأpH=10.3£®
[·ضخِ²آدë]Na
2SO
3?7H
2Oشعا؟ببدآ£¬ت§ب¥½ل¾§ث®£¬½ّ¶ّ·¢ةْءث·ض½â·´س¦£¨×شةيرُ»¯»¹ش·´س¦£©
[½»ء÷جضآغ]
£¨1£©تµرé²ظ×÷¢عضذ³ئء؟²ظ×÷ضءةظ½ّذذ
4
4
´خ£®
£¨2£©¸ّNa
2SO
3?7H
2O¼سببت±زھ¸ô¾ّ؟صئّ£¬اëزش¼ٍزھµؤخؤ×ض؛ح»¯ر§·½³جت½¸ّزشثµأ÷£®
±ـأâNa2SO3±»رُ»¯£¬2Na2SO3+O2=2Na2SO4
±ـأâNa2SO3±»رُ»¯£¬2Na2SO3+O2=2Na2SO4
£®
£¨3£©²آدëNa
2SO
3شع¸كخآا؟ببدآ·¢ةْ·ض½â·´س¦µؤ»¯ر§·½³جت½تا
£®
£¨4£©تµرé¢ظ؛ح¢غضذ£¬ء½´خ²âµأµؤبـز؛pHدà²îأ÷دش£¬ئن؛دہيµؤ½âتحتا
تµرé¢غµؤبـز؛ضذ؟ةؤـسذNa2S£¬دàح¬جُ¼دآ£¬S2-ث®½â³ج¶ب´َسعSO32-£¬بـز؛µؤ¼îذشا؟
تµرé¢غµؤبـز؛ضذ؟ةؤـسذNa2S£¬دàح¬جُ¼دآ£¬S2-ث®½â³ج¶ب´َسعSO32-£¬بـز؛µؤ¼îذشا؟
£®تµرé¢غضذبـز؛µؤpH=10.3µؤشہيتا£¨زشدà¹طµؤہë×س·½³جت½±يت¾£©
S
2-+H
2O

HS
-+OH
-S
2-+H
2O

HS
-+OH
-£®
£¨5£©اëؤمةè¼ئ¼ٍµ¥µؤتµرé·½°¸£¬¸ّةدتِ·ضخِ،¢²آدë¼°½âتحزش×ôض¤£®اë¼ٍµ¥ذًتِتµرé²½ضè،¢دضدَ؛ح½لآغ£®
ب،¢غبـز؛تتء؟سعتش¹ـضذ£¬دٍئنضذ¼سد،H2SO4£¬بçسذ¸¯µ°ئّخ¶ئّجهةْ³ة£¬شٍض¤أ÷سذS2-
ب،¢غبـز؛تتء؟سعتش¹ـضذ£¬دٍئنضذ¼سد،H2SO4£¬بçسذ¸¯µ°ئّخ¶ئّجهةْ³ة£¬شٍض¤أ÷سذS2-
،¢
ءيب،¢غضذبـز؛تتء؟سعتش¹ـضذ£¬¼سبëHClثل»¯µؤBaCl2£¬بçسذ°×ة«³ءµيةْ³ة£¬ض¤أ÷سذSO42-
ءيب،¢غضذبـز؛تتء؟سعتش¹ـضذ£¬¼سبëHClثل»¯µؤBaCl2£¬بçسذ°×ة«³ءµيةْ³ة£¬ض¤أ÷سذSO42-
£®




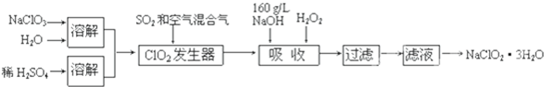
 HS-+OH-
HS-+OH- HS-+OH-
HS-+OH-