
���� ���ݳ�����Һ�ã�ԭ��Һû�����Ӧ�����ӣ�
��1����Һ��ǿ���ԣ�˵����Һ�п϶�����H+����H+��CO32-��Ӧ������Ӧ�����ܹ��棻
��2��CCl4����Ϻ�ɫ��˵����I2����������I-�����������������ģ��Ӷ�˵����Һ�к���I-������I-��Ӧ�����Ӳ��ܹ��棻
��3������ʵ�飨3��������Һ��������Ϊ���ԣ��ڵμӹ����к͵μ���Ϻ���Һ��������������Fe3+��Mg2+��Al3+����Ӧ����������������ì�ܣ�
��4��ȡ����������������Һ��Na2CO3��Һ���а�ɫ�������ɣ�˵����Һ�п϶�����Ba2+��
��5������3���õ��ļ�����Һ���ȣ�������ų�����������ʹʪ��ĺ�ɫʯ����ֽ����������Ϊ�������ݴ˽�ɣ�
��� �⣺��1����Һ��ǿ���ԣ�˵����Һ�п϶�����H+����H+��CO32-��Ӧ������Ӧ�����ܹ��棬˵����Һ�п϶�������CO32-��
��2��CCl4����Ϻ�ɫ��˵����I2����������I-�����������������ģ��Ӷ�˵����Һ�к���I-����I-��Fe3+��NO3-��H+�ܷ���������ԭ��Ӧ�������ܹ��棬˵����Һ�п϶�������Fe3+��NO3-��
��3������ʵ�飨3��������Һ��������Ϊ���ԣ��ڵμӹ����к͵μ���Ϻ���Һ��������������Fe3+��Mg2+��Al3+����Ӧ����������˵����Һ�п϶�������Fe3+��Mg2+��Al3+��
��4��ȡ����������������Һ��Na2CO3��Һ���а�ɫ�������ɣ�˵����Һ�п϶�����Ba2+����Ba2+����SO42-����������˵����Һ�в���SO42-��
��5������3���õ��ļ�����Һ���ȣ�������ų�����������ʹʪ��ĺ�ɫʯ����ֽ��������˵��һ������NH4+��
����������һ�����ڵ�������NH4+��Ba2+��I-��һ�������ڵ�������Mg2+��Al3+��Fe3+��SO42-��CO32-��NO3-��
�ʴ�Ϊ��NH4+��Ba2+��I-��Mg2+��Al3+��Fe3+��SO42-��CO32-��NO3-��
���� ���⿼�����ʵļ��鼰����Ϊ��Ƶ���㣬���ճ�������֮��ķ�Ӧ�����Ӽ����Ϊ�ƶϵĹؼ������ط������ƶ��������ۺϿ��飬��Ŀ�ѶȲ���


 ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д�
ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д� â���̸����������������ϵ�д�
â���̸����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 3��1 | B�� | 5��1 | C�� | 4��1 | D�� | 9��2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����Cu��OH��2����Һ | B�� | ��ˮ | C�� | ����KMnO4��Һ | D�� | FeCl3��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 5��3 | B�� | 5��4 | C�� | 1��1 | D�� | 3��5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NH4+��Fe3+��SO42-��SCN- | B�� | Na+��Mg2+��NO3-��OH- | ||
| C�� | K+��H+��Cl-��OH- | D�� | K+��H+��NO3-��CO32- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 75% | B�� | 13.6% | C�� | 20% | D�� | 25% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڢۢܢ� | B�� | �ڢۢ� | C�� | �ڢ� | D�� | �٢ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
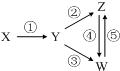 ���и��������У���һ��ʵ����ͼ��ʾ�١���ת����ϵ���ǣ�������
���и��������У���һ��ʵ����ͼ��ʾ�١���ת����ϵ���ǣ�������| X | Y | Z | W | |
| A | Fe3O4 | Fe | FeCl2 | FeCl3 |
| B | Al | Al2O3 | NaAlO2 | Al��OH��3 |
| C | H2SO4 | SO2 | S | SO3 |
| D | CH3CH2Br | CH2=CH2 | C2H5OH | CH2BrCH2Br |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com