
��12�֣�����ѧ�����л���ѧ������
������F�Ǻϳ���ũҩ����Ҫ�м��塣
�Ի�����A������ʽΪC7H7Cl��Ϊԭ�Ϻϳɻ�����F�Ĺ����������£�
��1����ӦA��B�Ļ�ѧ����ʽΪ_______��
��2��D�ķ���ʽΪ_______��
��3��������F�к��������ŵ�����Ϊ_________��B��C�ķ�Ӧ����Ϊ_______��
��4�� E��F��ת���У�������һ�ֺ�����Ԫ���ĸ���������F��Ϊͬ���칹�壬��ṹ��ʽΪ_______ ��
��5����ӦC��D�����У�D���ܷ���ˮ�⣬�����ڼ�����Լ��� _______��
��12�֣�
��1��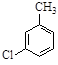 + Cl2
+ Cl2 
 + HCl��2�֣�
+ HCl��2�֣�
��2��C9H9O2Cl��2�֣�
��3�� �ʻ���2�֣� ȡ����Ӧ��2�֣�
��4�� 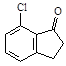 ��2�֣�
��2�֣�
��5��FeCl3��Һ����Ũ��ˮ���������ữ����������Һ����2�֣�
�������������������Ŀ������Ϣ��C�Ľṹ��ʽ��֪AΪ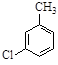 ���ڹ��������£���CH3��Cl2����ȡ����Ӧ�����ɵ�BΪ��
���ڹ��������£���CH3��Cl2����ȡ����Ӧ�����ɵ�BΪ�� ��������Ŀ������Ϣ����֪Cת����DΪ��
��������Ŀ������Ϣ����֪Cת����DΪ��
��1����ӦA��B���ڹ��������£���CH3��Cl2����ȡ����Ӧ�����Ի�ѧ����ʽΪ��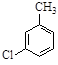 + Cl2
+ Cl2 
 + HCl
+ HCl
��2��D�Ľṹ��ʽΪ�� ���ɵ÷���ʽΪ��C9H9O2Cl
���ɵ÷���ʽΪ��C9H9O2Cl
��3������F�Ľṹ��ʽ��֪���еĺ���������Ϊ�ʻ����Ա�B��C�Ľṹ��ʽ��֪B��C�ķ�Ӧ����Ϊ��ȡ����Ӧ��
��4������һ�෴Ӧ�ɵú�����Ԫ���ĸ�������Խṹ��ʽΪ��
��5�����D����ˮ�ⷴӦ�������ϵ�Cl����OHȡ����������ǻ����Լ�Ϊ��FeCl3��Һ��Ũ��ˮ��Cl��ȡ��������ҺΪCl?��Ҳ�����������ữ����������Һ���顣
���㣺���⿼���л��ϳ��ƶϡ���Ӧ���͡�ͬ���칹�塢��ѧ����ʽ����д�����ʵļ��顣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��16�֣��Ի�����Ϊԭ��������������������к�Fe2O3��SiO2��Al2O3��MgO�ȣ����������Ʊ����죨Fe2O3���Ĺ������£�
��1�����ܹ�����Fe2O3��ϡ���ᷴӦ�Ļ�ѧ����ʽΪ ��
������A����Ҫ�ɷݵĻ�ѧʽΪ ��
��2����ԭ�����м���FeS2��Ŀ���ǽ���Һ�е�Fe3 +��ԭΪFe2 +��������������ΪH2SO4������ɸ÷�Ӧ�����ӷ���ʽ��FeS2 + 14Fe3 + + H2O="=" 15Fe2 + + SO42- + ��
��3�����������У�O2��NaOH��Fe2+��Ӧ�����ӷ���ʽΪ ��
��4��Ϊ��ȷ�����������������������Ҫ������Һ��pH�ķ�Χ�� ���������ӳ�����pH���±�������ҺB���Ի��յ������У�д��ѧʽ�� ��
| ������ | Fe(OH)3 | Al(OH)3 | Fe(OH)2 | Mg(OH)2 |
| ��ʼ����pH | 2.7 | 3.8 | 7.6 | 9.4 |
| ��ȫ����pH | 3.2 | 5.2 | 9.7 | 12.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
X��Y��Z��W��Q����Ԫ��ԭ��������������XΪ�ؿ��к�����ߵ�Ԫ�أ������ڱ���Y��X��Z��Q���ڣ�Q��X����ܲ��ϵĵ�������ͬ��Wԭ�Ӻ��������ֲ�ͬ�ܼ��ĵ��ӣ�������ܼ���û��δ�ɶԵ��ӣ�W��X���γ�W2X��WX���ֻ����
�ش��������⣺
��1��X����ԭ��������С��Ԫ���γ�ԭ�Ӹ�����Ϊ1��1�ķ��ӣ��÷��ӵĵ���ʽΪ ��
��2��W2+�ĺ�������Ų�ʽΪ ��
��3��Z����������ˮ��ˮҺ�� ɫ��������ͨ��Y��ij���������Һ��ɫ��ȥ���û�ѧ����ʽ��ʾԭ�� ��
��4��Y��ZԪ�صĵ�һ������Y Z���>������<����=������ X��ؿ��к����ڶ���Ԫ���γɵĻ����������ľ�������Ϊ ��
��5����֪X�ֱ���Ԫ��̼�����γɻ����������·�Ӧ��
2CX��g��+X2(g)=2CX2(g) ��H=��566.0kJ��mol-1
N2(g)+X2(g)="2NX(g)" ��H=189.5kJ��mol-1
2NX(g)+X2(g)=2NX2(g) ��H=��112.97kJ��mol-1
д��NX2��CX��Ӧ���ɴ����д��ڵ�������̬���ʵ��Ȼ�ѧ����ʽ�� ��
��6��Y����ԭ���γ�P4Y3���ӣ��÷�����û�Цм����Ҹ�ԭ���������Ѵ�8���ӽṹ����һ��P4Y3�����к��еļ��Լ��ͷǼ��Լ��ĸ����ֱ�Ϊ ���� ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
���ͱ����ǰ���ҩ�ijɷ֣���ѧʽΪC12H12N2O3�����ӽṹ����������Ԫ������ͼ����AΪԭ�Ϻϳɱ��ͱ�������ʾ��ͼ�����������ա�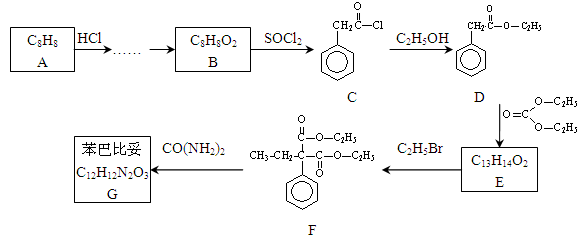
��֪�����л���D��E���Ǽ�����CH2��������ԭ�����ʻ�Ӱ����Խϸߣ����������·�Ӧ��
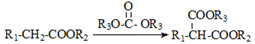
��
��1��������A��HCl��Ӧ����ȡB������з�Ӧ����������Ϊ ��
��2��һ������B��Ϊͬ���칹�壬�ұ�����ֻ��һ��ȡ����������ͬ���칹��
�� �֣�д������һ�ֽṹ��ϵͳ���� ��
��3��д��D ת��ΪE�Ļ�ѧ����ʽ��
��4�����ͱ���G�Ľṹ��ʽ��
��5��E��CO(NH2)2��һ�������ºϳɵĸ߷��ӽṹ��ʽ��
��6����֪�� ������ƺ���������B��ͬϵ��
������ƺ���������B��ͬϵ�� Ϊԭ��
Ϊԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��10�֣���ú��ʯ���п�������������ԭ��A��B��A��һ�ֹ�ʵ����������IJ�����������һ�����ҵ�ʯ�ͻ�����չˮƽ��B��һ�ֱ�ˮ�����״Һ̬����0��1mol��������������������ȫȼ�գ�����0��6molCO2��0��3molˮ���ش��������⣺
��1��A�ĵ���ʽ___ ____ ��B�Ľṹ��ʽ______ _��
��2����A���ڵ�ͬϵ��Cʹ������Ȼ�̼��Һ��ɫ�Ļ�ѧ��Ӧ����ʽ��
______ ______ _����Ӧ���ͣ�______ ��
��3���ڵ�ˮ�м���B���ʵ�����
��4��B��Ũ�����Ũ������50��60�淴Ӧ�Ļ�ѧ��Ӧ����ʽ��
____ _����Ӧ���ͣ�___ _ _��
��5����������A��B��ȫȼ��ʱ����O2�����ʵ���________���A>B������A<B����A��B������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
(15 ��)
���ĵð������������ಡ��ҩ�����������һ�ֺϳ�·��(���巴Ӧ�����Ͳ����Լ���)��
�ش��������⣺
��1���Լ�a�� ���Լ�b�Ľṹ��ʽΪ ��b�й����ŵ������� ��
��2���۵ķ�Ӧ������ ��
��3���ĵð��ķ���ʽΪ ��
��4���Լ�b���ɱ��龭������Ӧ�ϳɣ�
��Ӧ1���Լ�������Ϊ ��
��Ӧ2�Ļ�ѧ����ʽΪ ��
��Ӧ3�ķ�Ӧ������ ��(����������Ҳ����)
��5�����㻯����D��1�����ӵ�ͬ���칹�壬������������������ţ��ܷ���������Ӧ��D�ܱ�KMnO4������Һ������E( C2H4O2) �ͷ��㻯����F (C8H6O4)��E��F��̼��������Һ��Ӧ���ܷų�CO2���壬F�����ϵ�һ��������ֻ��һ�֡�D�Ľṹ��ʽΪ ��
��F����һ��������Ļ�ѧ����ʽΪ ��
��������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��12�֣�����ѧ�����л���ѧ������
����������һ�������������˷ܼ�����·�ߺϳ���ͼ��ʾ��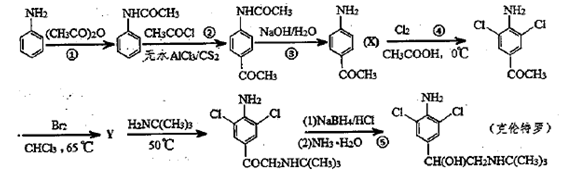
��1���������ķ���ʽΪ ��
��2����Ӧ�ܡ��ݵķ�Ӧ���ͷֱ��� �� ��
��3����Ӧ�۵Ļ�ѧ��Ӧ����ʽΪ ��
��4��Y�Ľṹ��ʽ�� ��
��5�����б����� NHһ���ܷ���������Ӧ��X��ͬ���칹���� �֡�
NHһ���ܷ���������Ӧ��X��ͬ���칹���� �֡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��14�֣� �л���A1��A2�ֱ��Ũ������һ���¶��¹���ֻ������B��B�������ܶ���ͬ��ͬѹ��H2�ܶȵ�59�����ڴ��������£�1mol B���Ժ�4mol H2�����ӳɷ�Ӧ��B��һԪ��������������(ͬ������)���й�����֮���ת����ϵ������F�ķ���ʽΪC9H10O3�����£�
�л���A1��A2�ֱ��Ũ������һ���¶��¹���ֻ������B��B�������ܶ���ͬ��ͬѹ��H2�ܶȵ�59�����ڴ��������£�1mol B���Ժ�4mol H2�����ӳɷ�Ӧ��B��һԪ��������������(ͬ������)���й�����֮���ת����ϵ������F�ķ���ʽΪC9H10O3�����£�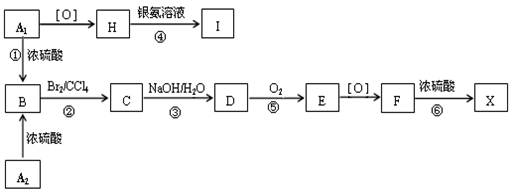
��ش��������⣺
��1�� ��Ӧ�� �� ��������ȡ����Ӧ���ǣ�_______________��
��2�� д��A2��F�����ʵĽṹ��ʽ��A2 ______________��F_____________________��
��3�� д���ۡ���������Ӧ�Ļ�ѧ����ʽ��
_______________________________________________________________��
_______________________________________________________________��
��4�� ������E�ж���ͬ���칹�壬�������������Ҿ���������λ������ͬ���칹��Ľṹ��ʽ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�������������۾����ϵľۺ���E�ǣ�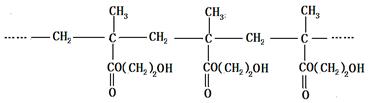
һ�ֺϳɾۺ���E��·�����£�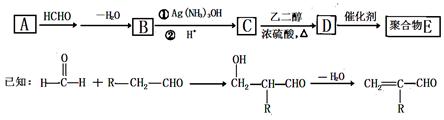
�ش��������⣺
��1��A��������Cu(OH)2����Һ��Ӧ����ש��ɫ������A�Ľṹ��ʽ�� ��
��2��D�к��еĹ���������Ϊ ��
��3��D��E�ķ�Ӧ������ ��Ӧ��
��4��C�ж���ͬ���칹�塣�������Һ���̼̼˫����ͬ���칹�干�� ��(������˳���칹)��д�����к˴Ź����������֮��Ϊ1��1��1��3��ͬ���칹��Ľṹ��ʽ ��
��5��д������ϩ�ϳ��Ҷ����Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com