
| m |
| M |
| 32% | ||
|
| 120kg��8��64 |
| 4��120 |
| 128��1000g |
| 64g/mol |


 �����Ļ�������ҵϵ�д�
�����Ļ�������ҵϵ�д� ����������������ϵ�д�
����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | ��� | ��ѧ������ | ˮ��Һ�Ƿ� | ����̬�Ƿ� | �Ƿ�Ϊ����� | |
| A | NaOH | ǿ�� | ���Ӽ� | ���� | ���� | �� |
| B | HCl | ���ۻ����� | �Ǽ��Լ� | ���� | ���� | �� |
| C | Na2S | ���ӻ����� | ���Ӽ��ͷǼ��Լ� | ���� | ���� | �� |
| D | CO2 | ������ | ���ۼ� | ���� | ������ | �� |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���е�ߵͣ�HF��HCl��HBr��HI |
| B���뾶��С��F-��Cl-��Br-��I- |
| C����ԭ�Դ�С��Li��Na��K��Rb |
| D�����������٣�1H��2H��3H |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
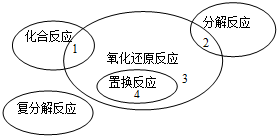
| A��4Fe��OH��2+O2+2H2O�T4Fe��OH��3 | ||||
B��2NaHCO3
| ||||
C��CH4+2O2
| ||||
| D��Cl2+2KBr�TBr2+2KCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��O2 |
| B��N2 |
| C��H2S |
| D��O3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �ơ�þ������ͭ���仯�����ڿ��к������������й㷺��Ӧ�ã�
�ơ�þ������ͭ���仯�����ڿ��к������������й㷺��Ӧ�ã��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com