



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 Fe(SCN)2+ K1="200" ��Fe(SCN)2++SCN-
Fe(SCN)2+ K1="200" ��Fe(SCN)2++SCN- Fe(SCN)2+ K2
Fe(SCN)2+ K2�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Fe(NO3)2ϡ��Һ�м������3Fe2����4H����NO3��===3Fe3����NO����2H2O |
B�����Ȼ���Ũ��Һ�����ˮ�У���ȡ������������Fe3����3H2O Fe(OH)3����3H�� Fe(OH)3����3H�� |
C��̼�������Һ��������NaOH��Һ��Ϻ���ȣ�NH4����OH�� NH3����H2O NH3����H2O |
| D����Ƭ������������Һ��Ӧ��Al��2OH����AlO2����H2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��������ĵ��룺H2SO3 2H++ SO3 2H++ SO3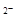 |
B���Ȼ��ˮ������ӷ���ʽΪ��NH4+ +H2O  NH3��H2O + H+ NH3��H2O + H+ |
C��NaHCO3ˮ������ӷ���ʽΪ��HCO3����H2O CO3 CO3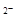 ��H3O+ ��H3O+ |
D�����Ȼ�����Һ�м�������İ�ˮ��A1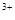 +3NH3��H2O= Al(OH)3��+3NH4+ +3NH3��H2O= Al(OH)3��+3NH4+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��FeSO4��Һ��ϡ���ᡢ˫��ˮ��ϣ�2Fe2++H2O2+2H+=2Fe3++2H2O |
B����ʯī���缫���CuCl2��Һ��2Cl-+2H2O  2OH-+H2��+Cl2�� 2OH-+H2��+Cl2�� |
| C��ͭ��Ũ���ᷴӦ��Cu+4HNO3(Ũ)= Cu2++2NO3-+2NO��+2H2O |
| D��NaHSO4��Һ������Ba(OH)2��Һ��ϣ�2H++SO42-+Ba2++2OH-=BaSO4��+2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ʵ�����ô���ʯ��ϡ������ȡCO2��2H+ + CO32-= CO2 + H2O |
| B������ϡ���ᷴӦ��Fe + 2H+= H2 + Fe3+ |
| C��NH4HCO3��Һ�м��������NaOH��Һ��NH4+ + OH-= NH3+H2O |
| D��NaHCO3��Һ��NaOH��Һ��Ӧ��OH- + HCO3-= CO32- + H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Pt�缫���������CuC12��Һ��2H2O��Cu2����2Cl��ͨ��H2����C12����Cu(OH)2�� |
| B��0.01 mol/L NH4Al(SO4)2��Һ��0.02 mol/L Ba(OH)2��Һ�������ϣ�NH4+��Al3����2SO42-��2Ba2����4OH��=2BaSO4����Al(OH)3����NH3��H2O |
| C�����Ը��������Һ�еμ�H2O2��Һ4MnO4�� + 4H2O2 + 12H+= 4Mn2+ + 7O2��+ 10H2O |
| D��������������������Fe3O4��8H��=Fe2����2Fe3����4H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����������������Fe2O3��6H����2I����2Fe2����I2��3H2O |
| B��CuƬ����FeCl3��Һ�У�Cu��Fe3��=Fe2����Cu2�� |
C����Al2��SO4��3��Һ�м�������İ�ˮ��Al3+��4NH3·H2O= ��4 ��4 ��2H2O ��2H2O |
D����NaHSO4��Һ�еμ�Ba��OH��2��Һ�����ԣ�H���� ��Ba2����OH��=BaSO4����H2O ��Ba2����OH��=BaSO4����H2O |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com