
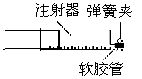
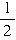 ��4���£�10��10-3L��=5.85mol/L��������������Ϊ0.048g/64g?mol-1��1/2��5��22400mL?mol-1=42mL��
��4���£�10��10-3L��=5.85mol/L��������������Ϊ0.048g/64g?mol-1��1/2��5��22400mL?mol-1=42mL�� 

 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

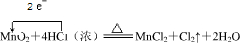
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
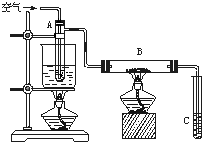 ij����С������ͼ��ʾ�����Ҵ��Ĵ�����ʵ��̽�����Թ�A��ʢ����ˮ�Ҵ���B��װ��CuO����ʯ���������壩���Թ�C�зŵ�������ˮ����ش��������⣺
ij����С������ͼ��ʾ�����Ҵ��Ĵ�����ʵ��̽�����Թ�A��ʢ����ˮ�Ҵ���B��װ��CuO����ʯ���������壩���Թ�C�зŵ�������ˮ����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

��1����ͼ��һ֧���Ϊ50 mL��ע��������������Ͳ����������ã����γ���ͷ������48 mgͭƬ��Ȼ���ڰ���ͷ�����������ܡ�
��2���ȳ�ȡ10 mol��L-1��ϡHNO3��������������Һ���У�Ŀ����______________________��
��3���ٳ�ȡ10 mL 6 mol��L-1��ϡHNO3���ѵ��ɼм����������ϣ���Ӧ��ʼʱ���������ӿ졣Ԥ����ע�����пɹ۲쵽��Щ����������д����
��4����Ӧ��Ϻ�ֹˮ�У���������������36.2 mL�����кõ��ɼУ��۲쵽___________����ҡע�������£�����Ƭ���ֹ۲쵽_________________________���ظ����ϲ������Σ�����Ͳ�������NOΪֹ����Ͳ����Һ��c��![]() ��Ϊ___________��c��H+��Ϊ___________��
��Ϊ___________��c��H+��Ϊ___________��
��5����ʵ�����������ŵ���_________________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com