
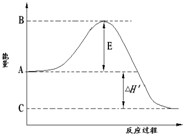
25 ��ʱ���ϳɰ���Ӧ���Ȼ�ѧ����ʽΪ��
N2 (g) +3H2 (g) 2NH3(g) ��H����92.4 kJ/mol
����������ȷ����
A������������������ʱ��˵���÷�Ӧһ���ﵽƽ��״̬
B�����������������ԭ���Ķ�������(��)��С����(��)����ƽ�������ƶ�
C�������������N2��ת���ʣ��������̴ﵽƽ������ʱ�䣬�������Ч��
D�����ܱ������з���1 mol N2��3 mol H2���з�Ӧ����÷�Ӧ�ų�������С��92.4 kJ
D?
����:A�������Ƿ�Ӧ�ﻹ��������������壬����˵�������Ƿ�ﵽƽ�⣬�����������������䡣
B�����������������ԭ���Ķ�������Ӧ�������Ũ�Ⱦ���С�������(��)����(��)����С��ƽ�������ƶ�
C�������Ȳ������N2��ת����,�������̴ﵽƽ������ʱ�䣬�������Ч��
D�����ڴ˷�Ӧʱ���淴Ӧ�����ܱ������з���1 mol N2��3 mol H2���з�Ӧ����÷�Ӧ�ų�������С��92.4 kJ


 ������ÿ�ʱ�Ż���ҵϵ�д�
������ÿ�ʱ�Ż���ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �ϳɰ���ҵ�Ǽ�Ϊ��Ҫ�Ļ�ѧ��ҵ������Ժϳɰ���ҵ���о���Ȼ������������ϳ�ԭ��Ϊ��N2��g��+3H2��g��?2NH3��g������H=-92.2kJ/mol���ش��������⣺
�ϳɰ���ҵ�Ǽ�Ϊ��Ҫ�Ļ�ѧ��ҵ������Ժϳɰ���ҵ���о���Ȼ������������ϳ�ԭ��Ϊ��N2��g��+3H2��g��?2NH3��g������H=-92.2kJ/mol���ش��������⣺| 1 |
| 3 |
| 10 | 20 | 30 | 60 | |
| 300 | 52.0 | 64.2 | 71.0 | 84.2 |
| 400 | 25.1 | 38.2 | 47.0 | 65.2 |
| 500 | 10.6 | 19.1 | 26.4 | 42.0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ������ʡ����У�����ڶ����������ۻ�ѧ�Ծ��������棩 ���ͣ������
��1����֪��O2 (g)= O2�� (g)+e�� ��H1= +1175.7 kJ��mol��1
PtF6(g)+ e��= PtF6��(g)???? ��H2= - 771.1 kJ��mol��1
O2+PtF6��(s)=O2+(g)+PtF6�� (g)?? ��H3=+482.2 kJ��mol��1
��Ӧ��O2��g��+ PtF6 (g) = O2+PtF6(s)����H=_____ kJ��mol-1��
��ͼΪ�ϳɰ���Ӧ��ʹ����ͬ�Ĵ�������ͬ�¶Ⱥ�ѹǿ�����½��з� Ӧ����ʼʱN2��H2�������Ϊ1:3ʱ��ƽ�������а������������

�� ��һ�����¶��£������������ܱ������г��뵪������������������Ӧ��������˵����Ӧ�ﵽƽ��״̬����??????? ��
a����ϵ��ѹǿ���ֲ���?? ???? b�����������ܶȱ��ֲ���
c��N2��H2�������Ϊ1:3????? d����������ƽ��Ħ����������
���ֱ���vA��NH3����vB��NH3����ʾ�ӷ�Ӧ��ʼ��ƽ��״̬A��Bʱ�ķ�Ӧ���ʣ���vA��NH3��??? vB��NH3��������>������<������=�������÷�Ӧ�ĵ�ƽ�ⳣ��kA ??? kB������>������<������=��������250 ����1.0��104kPa�´ﵽƽ�⣬H2��ת����Ϊ????? %������������С�����һλ����
��3��25��ʱ����a mol NH4NO3����ˮ����Һ�����ԣ�ԭ��???????????????????????? ?????????? �������ӷ���ʽ��ʾ���������Һ�м���bL��ˮ����Һ�����ԣ������Ӱ�ˮ��Ũ��Ϊ?????????? mol/L���ú�a��b�Ĵ���ʽ��ʾ��NH3��H2O�ĵ���ƽ�ⳣ��ΪKb=2��10-5��
��4����ͼ��ʾ��װ����Ϊ����ȼ�ϵ�أ��������ҺΪKOH��Һ����ͨ��װ����ʵ�������϶�ͭ�����һ��ʱ���װ��������Һ��pH ???? �����������������С����������������a���缫��Ӧ����ʽΪ????????????????? ������ƽ�������װ���������������仯��25.6g����Һ������ͭ��ʣ�ࣩ����װ���������������ļ���????? L����״���£���

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com