
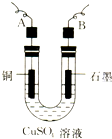
 ƒ≥—ß…˙”√µÁΩ‚¥ø檵ƒCuSO4»Ð“∫µƒ∑Ω∑®£¨≤¢∏˘æðµÁº´…œŒˆ≥ˆCuµƒ÷ ¡ø£®m g£©“‘º∞µÁº´…œ≤˙…˙∆¯ÃµƒÃª˝£®V mL ±Í◊º◊¥øˆ£©¿¥≤‚∂®Cuµƒœý∂‘‘≠◊”÷ ¡ø£¨À˘”√≤ø∑÷“«∆˜»Áœ¬ÕºÀ˘ 棨ªÿ¥œ¬¡–Œ £∫
ƒ≥—ß…˙”√µÁΩ‚¥ø檵ƒCuSO4»Ð“∫µƒ∑Ω∑®£¨≤¢∏˘æðµÁº´…œŒˆ≥ˆCuµƒ÷ ¡ø£®m g£©“‘º∞µÁº´…œ≤˙…˙∆¯ÃµƒÃª˝£®V mL ±Í◊º◊¥øˆ£©¿¥≤‚∂®Cuµƒœý∂‘‘≠◊”÷ ¡ø£¨À˘”√≤ø∑÷“«∆˜»Áœ¬ÕºÀ˘ 棨ªÿ¥œ¬¡–Œ £∫∑÷Œˆ £®1£©µÁΩ‚¡ÚÀ·Õ≠»Ð“∫£¨—Ùº´«‚—ı∏˘¿Î◊” ßµÁ◊”£¨“ıº´Õ≠¿Î◊”µ√µÁ◊”£ª
£®2£©∏˘æðµÁº´…œŒˆ≥ˆÕ≠µƒ÷ ¡ø“‘º∞µÁº´…œ≤˙…˙∆¯ÃµƒÃª˝¿¥≤‚∂®Õ≠µƒœý∂‘‘≠◊”÷ ¡ø£¨‘Ú–Ë“™≥∆¡øµÁΩ‚«∞∫ÛµÁº´µƒ÷ ¡ø£ª
£®3£©∏˘æðµÁ◊” ÿ∫„ø…÷™£¨2Cu°´02°¸£¨“‘¥Àº∆À„£Æ
Ω‚¥ Ω‚£∫£®1£©µÁΩ‚¡ÚÀ·Õ≠»Ð“∫£¨—Ùº´«‚—ı∏˘¿Î◊” ßµÁ◊”£¨“ıº´Õ≠¿Î◊”µ√µÁ◊”£¨∆‰µÁΩ‚∑¥”¶µƒ¿Î◊”∑Ω≥Ã ΩŒ™2Cu2++2H20$\frac{\underline{\;µÁΩ‚\;}}{\;}$2Cu+4H++02°¸£¨
π ¥∞∏Œ™£∫2Cu2++2H20$\frac{\underline{\;µÁΩ‚\;}}{\;}$2Cu+4H++02°¸£ª
£®2£©∏˘æðµÁº´…œŒˆ≥ˆÕ≠µƒ÷ ¡ø“‘º∞µÁº´…œ≤˙…˙∆¯ÃµƒÃª˝¿¥≤‚∂®Õ≠µƒœý∂‘‘≠◊”÷ ¡ø£¨‘Ú–Ë“™≥∆¡øµÁΩ‚«∞∫ÛµÁº´µƒ÷ ¡ø£¨
A£Æ µ—È÷Æ«∞”¶≥∆¡øµÁΩ‚«∞µÁº´µƒ÷ ¡ø£¨π ’˝»∑
B£ÆµÁΩ‚∫Û£¨µÁº´‘⁄∫Ê∏…≥∆÷ÿ«∞£¨±ÿ–Δ√’Ù¡ÛÀÆ≥Âœ¥£¨ºı…ŸŒÛ≤Ó£¨π ’˝»∑£ª
C£ÆπŒœ¬µÁΩ‚∫ÛµÁº´…œŒˆ≥ˆµƒÕ≠£¨≤¢«Âœ¥°¢≥∆÷ÿ£¨≤Ÿ◊˜≤ªæ´»∑£¨ƒ—µ√µΩ◊º»∑µƒCuµƒ÷ ¡ø£¨π ¥ÌŒÛ£ª
D£ÆµÁº´‘⁄∫Ê∏…≥∆÷ÿµƒ≤Ÿ◊˜÷–±ÿ–Î∞¥°∞∫Ê∏…-≥∆÷ÿ-‘Ÿ∫Ê∏…-‘Ÿ≥∆÷ÿ°±Ω¯––£¨∑¿÷πCu±ª—ıªØ£¨π ’˝»∑£ª
E£Æ‘⁄”–ø’∆¯¥Ê‘⁄µƒ«Èøˆœ¬£¨∫Ê∏…µÁº´±ÿ–Î≤…”√µÕŒ¬∫Ê∏…µƒ∑Ω∑®£¨∑¿÷πCu±ª—ıªØ£¨π ’˝»∑£ª
π ¥∞∏Œ™£∫ABDE£ª
£®3£©…ËCuµƒœý∂‘‘≠◊”÷ ¡øŒ™x£¨
∏˘æðµÁ◊” ÿ∫„ø…÷™£¨2Cu°´02°¸£¨
2x 1
ng $\frac{V°¡1{0}^{-3}L}{22.4L/mol}$
‘Ú 2x°¡$\frac{V°¡1{0}^{-3}L}{22.4L/mol}$=n£¨
Ω‚µ√£∫x=$\frac{11200m}{V}$£¨
π ¥∞∏Œ™£∫$\frac{11200m}{V}$£Æ
µ„∆¿ ±æÂøº≤È¡ÀµÁΩ‚‘≠¿Ìµƒ”¶”√º∞œý∂‘‘≠◊”÷ ¡øµƒ≤‚∂®£¨◊¢“‚µÁΩ‚÷–µÁ◊” ÿ∫„º¥ø…Ω‚¥£¨Ã‚ƒøƒ—∂»÷–µ»£¨≤ý÷ÿ”⁄øº≤È—ß…˙µƒ∑÷ŒˆƒÐ¡¶∫Õº∆À„ƒÐ¡¶£Æ


 ‘ƒ∂¡øÏ≥µœµ¡–¥∞∏
‘ƒ∂¡øÏ≥µœµ¡–¥∞∏
| ƒÍº∂ | ∏þ÷–øŒ≥à | ƒÍº∂ | ≥ı÷–øŒ≥à |
| ∏þ“ª | ∏þ“ª√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥ı“ª | ≥ı“ª√‚∑—øŒ≥ÃÕ∆ºˆ£° |
| ∏þ∂˛ | ∏þ∂˛√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥ı∂˛ | ≥ı∂˛√‚∑—øŒ≥ÃÕ∆ºˆ£° |
| ∏þ»˝ | ∏þ»˝√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥ı»˝ | ≥ı»˝√‚∑—øŒ≥ÃÕ∆ºˆ£° |
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫—°‘ÒÂ
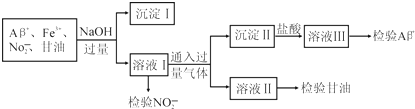
| A£Æ | Õ®»Îµƒπ˝¡ø∆¯ÃÂø…ƒÐ «CO2 | |
| B£Æ | ∑÷¿Î≥ˆ≥¡µÌ¢Òµƒ≤Ÿ◊˜√˚≥∆ «π˝¬À£¨¿˚”√≥¡µÌ¢Òø…ºÏ—È≥ˆFe3+ | |
| C£Æ | »ÙœÚ»Ð“∫¢Û÷–œ»º”◊„¡øNH4F∫Û‘Ÿº”∞±ÀÆø…“‘ºÏ—ÈAl3+ | |
| D£Æ | ”√À·–‘KI»Ð“∫ºÏ—ÈNO2-¿Î◊”µƒ¿Î◊”∑Ω≥Ã ΩŒ™2NO2-+2I-+4H+®T2NO+I2+2H2O |
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫—°‘ÒÂ
| A£Æ | pH=2µƒHA»Ð“∫”ÎpH=12µƒMOH»Ð“∫“‘»Œ“‚±»ªÏ∫œc£®H+£©+c£®M+£©=c£®OH-£©+c£®A-£© | |
| B£Æ | Na2CO3»Ð“∫£∫c£®OH-£©=c£®HCO3-£©+c£®H+£©+2c£®H2CO3£© | |
| C£Æ | “Œ¬œ¬pH=7µƒCH3COOH”ÎCH3COONaµƒªÏ∫œ“∫÷–¿Î◊”µƒ≈®∂»¥Û–°À≥–ÚŒ™£∫c£®Na+£©=c£®CHCOO-£©£æc£®H+£©=c£®OH-£© | |
| D£Æ | pH=4µƒNaHA»Ð“∫£∫c£®HA-£©£æc£®H+£©£æc£®H2A£©£æc£®A2-£© |
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫—°‘ÒÂ
| A£Æ | æ∆æ´ | B£Æ | ““À· | C£Æ | Àƒ¬»ªØú | D£Æ | ∆˚”Õ |
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫—°‘ÒÂ
| A£Æ | »Ð“∫÷–µƒSO42-œÚ’˝º´‘À∂Ø | B£Æ | µÁ◊”Õ®π˝µºœþ”…Õ≠∆¨¡˜œÚ–ø∆¨ | ||
| C£Æ | ’˝º´”–O2“ð≥ˆ | D£Æ | Õ≠∆¨…œ”–H2“ð≥ˆ |
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫Ω‚¥Ã‚
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫—°‘ÒÂ
| A£Æ | ≥Œ«Âµƒ ت“ÀƔΜ°—ŒÀ·∑¥”¶£∫OH-+H+=H2O | |
| B£Æ | «‚—ıªØ±µ»Ð“∫”Îœ°¡ÚÀ·∑¥”¶£∫Ba2++OH-+SO${\;}_{4}^{2-}$+H+=BaSO4°˝+H2O | |
| C£Æ | —ıªØÕ≠”ΗŒÀ·∑¥”¶£∫O2-+2H+=H2O | |
| D£Æ | ¬»∆¯”ÎÀÆ∑¥”¶£∫Cl2+H2O=2H++Cl-+ClO- |
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫—°‘ÒÂ
| A£Æ | Na+°¢Mg2+°¢Al3+ | B£Æ | HCl°¢H2S°¢Ar | C£Æ | H2O°¢OH-°¢Na+ | D£Æ | NH4+°¢Na+°¢F- |
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫—°‘ÒÂ
| A£Æ | º”ÀÆ’Òµ¥£¨π€≤Ï «∑Ò”–∑÷≤„œ÷œÛ | |
| B£Æ | º”““¥º’Òµ¥£¨π€≤Ï «∑Ò∑¢…˙∑÷≤„ | |
| C£Æ | º”–¬÷∆Cu£®OH£©2–¸◊«“∫£¨÷Û∑–£¨π€≤Ï”–ŒÞ◊©∫Ï…´≥¡µÌ…˙≥… | |
| D£Æ | º”»Î∫¨”–∑”ÙµƒNaOH»Ð“∫£¨º”»»£¨π€≤Ï∫Ï…´ «∑Ò±‰«≥ |
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
π˙º —ß–£”≈—° - ¡∑œ∞≤·¡–±Ì - ‘¡–±Ì
∫˛±± °ª•¡™Õ¯Œ•∑®∫Õ≤ª¡º–≈œ¢æŸ±®∆Ωî | Õ¯…œ”–∫¶–≈œ¢æŸ±®◊®«¯ | µÁ–≈’©∆≠柱®◊®«¯ | …Ê¿˙ ∑–ÈŒÞ÷˜“”–∫¶–≈œ¢æŸ±®◊®«¯ | …Ê∆Û«÷»®æŸ±®◊®«¯
Œ•∑®∫Õ≤ª¡º–≈œ¢æŸ±®µÁª∞£∫027-86699610 柱®” œ‰£∫58377363@163.com