
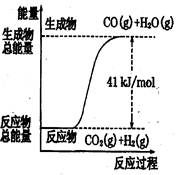
������ͼ��ʾ���Ȼ�ѧ����ʽ�� �� ��
A��CO+H2O=CO2+H2����H=+41 kJ/mol
B��CO(g)+H2O(g)=CO2(g)+H2(g);����H= −41 kJ/mol
C��CO2(g)+H2(g)=CO(g)+H2O(g)����H=+41 kJ/mol
D��CO2(g)+H2(g)=CO(g)+H2O(g)����H=−41kJ/mol
������������ͼ����жϷ�Ӧ������������������������������Ӧ�����ȷ�Ӧ��B��D����ȷ����ѡ��A��û�б������ʵ�״̬���Ǵ���ģ�������ȷ�Ĵ���C��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��1��������һ�ָ�Ч��������Դ��0.25mol������ȫȼ������Һ̬ˮʱ���ų�222.5kJ�����������ȼ���ȵ��Ȼ�ѧ��Ϊ
��1��������һ�ָ�Ч��������Դ��0.25mol������ȫȼ������Һ̬ˮʱ���ų�222.5kJ�����������ȼ���ȵ��Ȼ�ѧ��Ϊ| 1 |
| 2 |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�갲��ʡ�����ظߺ���ѧ�߶���ѧ�ڵڶ����¿���ѧ�Ծ����������� ���ͣ���ѡ��
������ͼ��ʾ���Ȼ�ѧ����ʽ�� �� ��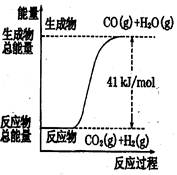
| A��CO+H2O=CO2+H2����H="+41" kJ/mol |
| B��CO(g)+H2O(g)=CO2(g)+H2(g);����H= ?41 kJ/mol |
| C��CO2(g)+H2(g)=CO(g)+H2O(g)����H="+41" kJ/mol |
| D��CO2(g)+H2(g) =CO(g)+H2O(g)����H=?41 kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�찲��ʡ�߶���ѧ�ڵڶ����¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
������ͼ��ʾ���Ȼ�ѧ����ʽ�� �� ��
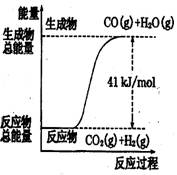
A��CO+H2O=CO2+H2����H=+41 kJ/mol
B��CO(g)+H2O(g)=CO2(g)+H2(g);����H= −41 kJ/mol
C��CO2(g)+H2(g)=CO(g)+H2O(g)����H=+41 kJ/mol
D��CO2(g)+H2(g) =CO(g)+H2O(g)����H=−41 kJ/mol
������������ͼ����жϷ�Ӧ������������������������������Ӧ�����ȷ�Ӧ��B��D����ȷ����ѡ��A��û�б������ʵ�״̬���Ǵ���ģ�������ȷ�Ĵ���C��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com