
����һ����Ҫ�Ļ�����Ʒ���ǵ��ʹ�ҵ���л��ϳɹ�ҵ�Լ��������ᡢ��κʹ���ȵ�ԭ�ϡ�
��1����Ϊȼ�Ͽ���������찱ȼ�ϵ�أ����������Ⱦ���ںܶ�����õ��㷺Ӧ�á����缫���Ͼ�Ϊ���Ե缫��KOH��Һ���������Һ����õ�ظ����缫��ӦʽΪ ��
��2����һ���¶��£��ڹ̶�������ܱ������н��п��淴Ӧ��N2+3H2 2NH3���ÿ��淴Ӧ�ﵽƽ��ı�־��________________��
2NH3���ÿ��淴Ӧ�ﵽƽ��ı�־��________________��
A��3v(H2)��=2v(NH3)��
B����λʱ������m mol N2��ͬʱ����3m mol H2
C�������ڵ���ѹǿ������ʱ����仯
D�����������ܶȲ�����ʱ��仯
E��a molN��N�����ѵ�ͬʱ����6amolN—H������
F��N2��H2��NH3�ķ�����֮��Ϊ1��3��2
��3��ij��ѧ�о���ѧϰС��ģ�ҵ�ϳɰ��ķ�Ӧ�����ݻ��̶�Ϊ2L���ܱ������ڳ���1molN2��3molH2��������ʴ�����������Ժ��Բ��ƣ�����һ���¶�ѹǿ�¿�ʼ��Ӧ������ѹ���Ƽ��������ѹǿ�ı仯���£�
| ��Ӧʱ��/min | 0 | 5 | 10 | 15 | 20 | 25 | 30 |
| ѹǿ/MPa | 16.80 | 14.78 | 13.86 | 13.27 | 12.85 | 12.60 | 12.60 |
��ӷ�Ӧ��ʼ��25minʱ����N2��ʾ��ƽ����Ӧ����= �����¶���ƽ�ⳣ��K= ��
��4����CO2��NH3Ϊԭ�Ϻϳ�����[��ѧʽΪCO(NH2)2]����Ҫ��Ӧ���£���֪��
��2NH3(g)+CO2(g) == NH2CO2 NH4(s) ��H= —l59.5 kJ·mol-1
��NH2CO2NH4(s)  CO(NH2)2(s)+H2O(g) ��H=+116.5 kJ·mol-1
CO(NH2)2(s)+H2O(g) ��H=+116.5 kJ·mol-1
��H2O(1) == H2O(g) ��H=+44.0kJ·mol-1
д��CO2��NH3�ϳ����غ�Һ̬ˮ���Ȼ�ѧ��Ӧ����ʽ ��
����������Ӧ�����ܱ������н�����NH2CO2NH4������300K�·ֽ⣬ƽ��ʱP[H2O(g)]Ϊa Pa������Ӧ�¶Ȳ��䣬����ϵ���������50%����P[H2O(g)]��ȡֵ��Χ��_________________���ú�a��ʽ�ӱ�ʾ��������ѹ=��ѹ�����ʵ���������
��֪ʶ�㡿��ѧƽ�� G1 G2 G3 G4 G5
���𰸽�����24����1��2NH3—6e—+6OH—=N2+6H2O 3��
��2��BCE 3��
��3��0.01 mol/(L.min) 2�� 2.37( mol/L)-2 2��
��4��2NH3(g)+CO2(g)== CO(NH2)2(s) +H2O(l) ��H= — 87.0 kJ·mol-1 2��
2a/3��P[H2O(g)]��a 2��
��������1��ȼ�ϵ��ȼ��Ϊ����������������Ӧ
��2��A������ʾ�����������ʣ���A����B����ȷ��C����Ӧ�����У���������仯ѹǿ�仯����һ������ﵽƽ�⣬C��ȷ��D��m���䣬V���䣬���ܶȲ��䣬����ƽ���־����D����E����ȷ��F��ƽ���־�Ǹ����Ũ�Ȳ��䣬��F����ѡ��BCE
��3����3��ʽ 2L ���ʵ���mol N2+3H2 2NH3
2NH3
��ʼ���ʵ���1 3 0
�仯�� x 3x 2x
ƽ���� 1-x 3-3x 2x
25min��0minѹǿ��Ϊ12.6/16.8=��4-2x��/4��x=0.5mol����N2��ʾ��ƽ����Ӧ����=0.5mol��2L��25min=0.01 mol/(L.min)��ƽ�ⳣ��K=c2��������/c3(����)��c��������= 2.37( mol/L)-2
��4��2NH3(g)+CO2(g)== CO(NH2)2(s) +H2O(l) ��H=—l59.5 kJ·mol-1+116.5 kJ·mol-1-44.0kJ·mol-1= — 87.0 kJ·mol-1
��5������ϵ���������50%����P[H2O(g)]��Ϊ2a/3����ƽ���������ƶ���2a/3��P[H2O(g)]��a
��˼·�㲦���⿼����������ԭ��Ӧ����˹���ɡ���ѧƽ���֪ʶ�㣬����������ԭ��Ӧʵ�ʡ���˹�����ں�����ѧƽ����жϷ�����֪ʶ�������������Щ֪ʶ�㶼�Ǹ߿��ȵ㣬���������ڴ����ۺ����У�ע�����غ�˼�����������⣬��Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A�� B��C��D��E���ֶ�����Ԫ�أ�Ԫ��A����������������������ȣ�A��C���γ�A2C2��A2C���ֻ����B������������Ӧ��ˮ����������BA3���������ң�D+��C2-������ͬ�ĵ�������EԪ������ϼ�����ͻ��ϼ۴�����Ϊ6��
��ش���������:
(1) C��Ԫ�����ڱ��е�λ��Ϊ____________��д��BA3�ĵ���ʽ_______________��
(2)����Ԫ���У�ԭ�Ӱ뾶������____________________(дԪ�ط���)������������Ӧ��ˮ������������ǿ������__________________(д��ѧʽ)��
(3)�������ҵ�ˮ��Һ�����ԣ��������ӷ���ʽ��ʾ__________________��
(4) A��C��E����Ԫ�ذ�ԭ�Ӹ�����1:1:1��ɵĻ�������BA3��Ӧ����B�ĵ��ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����(����)
A��ǿ�������Һһ�������������Һ�ĵ�����ǿ
B��ǿ����ʵ�ϡ��Һ�в��������ʷ���
C��ǿ����ʶ������ӻ������������ʶ��ǹ��ۻ�����
D����ͬ���������ֻҪ���ʵ�����Ũ����ͬ������̶�Ҳ��ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����һ��NaOH������Ʒ���������������Na2CO3��NaCl���ʣ�ijͬѧ���к͵ζ��ķ������ⶨNaOH�Ĵ��ȡ���������£�
�ٳ�ȡa g��Ʒ������ƿ�У���ˮ����ʹ�������ܽ⡣
�������Һ�м���������BaCl2��Һ��ʹCO ������ȫ��
������ȫ��
�������û��Һ�е���2��3�η�ָ̪ʾ����Ȼ����c mol��L��1��������еζ���
�ܵ���Ӧ���յ�ʱ�����������������ΪV mL��
�ݼ�����Ʒ�Ĵ��ȡ�
�Իش����������
(1)�ζ��յ�ʱ��Һ��ɫ��α仯��__________________________________________��
(2)�ڢڲ������ɵ�BaCO3����δ���˳�����ֱ��������ζ����Ƿ���NaoH�ĺ����ⶨ���Ӱ��(��ǡ���)______��������___________________________________��
(3)�ڢڲ������ɵ�BaCO3����δ���˳�����ֱ��������ζ����ܷ���ü�����ָʾ��(��ǡ���)______��������________________________________________��
(4)��������ʵ�����ݣ��г�����NaOH���ȵı���ʽ
________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ŀǰ����̼���š����ܹ�ע��CO2�IJ�������Ч�������ó�Ϊ��ѧ���о�����Ҫ���⡣
(1)����β����������Ҫԭ��Ϊ2NO(g)+2CO(g) 2CO2(g)+N2(g)�����ܱ������з����÷�Ӧʱ��c(CO2)���¶�(T)�����������(S)��ʱ��(I)�ı仯������ͼ��ʾ��
2CO2(g)+N2(g)�����ܱ������з����÷�Ӧʱ��c(CO2)���¶�(T)�����������(S)��ʱ��(I)�ı仯������ͼ��ʾ��
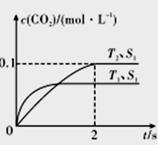
�ݴ��ж�:
�ٸ÷�Ӧ�ġ�H_____________0(�>����<��)��
����T2�¶��£�0-2s�ڵ�ƽ����Ӧ����v(N2) =____________mol/(L·s)��
�۵��������������һ��ʱ������������������ѧ��Ӧ���ʡ��������ı����S1>S2,�ڴ����Ӧͼ�л���c(CO2)��T1��S2�����´ﵽƽ������еı仯���ߡ�
�����÷�Ӧ�ھ��ȡ����ݵ��ܱ���ϵ�н��У�����ʾ��ͼ��ȷ����˵����Ӧ�ڽ��е�t1ʱ�̴ﵽƽ��״̬����_______________(�����)��
(2)ֱ���ŷ�úȼ�ղ������������������صĻ������⡣
��úȼ�ղ����������������������CH4����ԭNOx�������������������Ⱦ��
����:CH4(g)+2NO2(g)=N2(g)+CO2(g)+2H2O(g) ��H=-867kJ/mol
2NO2(g)=N2O4(g) ��H=-867kJ/mol
д��CH4(g)����ԭN2O4(g)����N2(g)��CO2(g)��H2O(g)���Ȼ�ѧ����ʽ:_____________________��
�ڽ�ȼú�����Ķ�����̼�������ã��ɴﵽ��̼�ŷŵ�Ŀ�ġ���ͼ��ͨ���˹�������ã���CO2(g)��H2O(g)Ϊԭ���Ʊ�HCOOH��O2��ԭ��ʾ��ͼ������b���淢���ĵ缫��ӦΪ_________________��
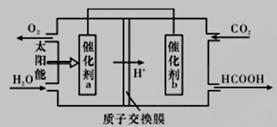
�۳�����0. 1 mol/L��HCOONa��ҺpHΪ10����HCOOH�ĵ��볣��Ka =_______________mol·L-1(��д���ռ�����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
FeCl3(aq)��KSCN(aq)���ʱ��������ƽ�⣺
Fe3+(aq)��SCN-(aq)  Fe(SCN)2+(aq)����֪ƽ��ʱ�����ʵ���Ũ��c[Fe(SCN)2+]���¶�T�Ĺ�ϵ��ͼ��ʾ��������˵����ȷ����
Fe(SCN)2+(aq)����֪ƽ��ʱ�����ʵ���Ũ��c[Fe(SCN)2+]���¶�T�Ĺ�ϵ��ͼ��ʾ��������˵����ȷ����
A��FeCl3(aq)��KSCN(aq)��Ӧ���Ȼ�ѧ��Ӧ����ʽΪ
Fe3��(aq)��SCN—(aq)  Fe(SCN)2��(aq)����H ��0
Fe(SCN)2��(aq)����H ��0
B���¶�ΪT1��T2ʱ����Ӧ��ƽ�ⳣ���ֱ�ΪK1��K2����K1��K2
C����Ӧ����D��ʱ��һ��������������
D��A����B����ȣ�A���c(Fe3��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ��ˮ�г�����һ������Cr2O ��CrO
��CrO �����ǻ����̬ϵͳ��ɺܴ�������л�ԭ�������dz��õ�һ�ִ����������������£�
�����ǻ����̬ϵͳ��ɺܴ�������л�ԭ�������dz��õ�һ�ִ����������������£�
CrO
 Cr2O
Cr2O
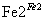 Cr3��
Cr3�� Cr(OH)3��
Cr(OH)3��
���еڢٲ��д���ƽ�⣺2CrO (��ɫ)��2H��
(��ɫ)��2H�� Cr2O
Cr2O (��ɫ)��H2O�������й�˵����ȷ����
(��ɫ)��H2O�������й�˵����ȷ����
A���ڢٲ���2v(Cr2O )��v(CrO
)��v(CrO )ʱ���ﵽ��ƽ��״̬
)ʱ���ﵽ��ƽ��״̬
B����������ƽ�⣬��������ϡ�������Һ��ɫ���ɫ����������CrO ������
������
C�������£�Cr��OH��3���ܶȻ�Ksp=10-32��Ҫʹc��Cr3+������10-5mol/L����Һ��pHӦ����9
D���ڢڲ��У���ԭ0.1 mol Cr2O ��Ҫ91.2 g FeSO4
��Ҫ91.2 g FeSO4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����
A.��ɢϵ�з�ɢ�����ӵ�ֱ����Fe(OH)3����Һ> Fe(OH)3����> FeCl3��Һ
B.���Է����еĻ�ѧ��Ӧ��һ���� H<0��
H<0�� S>0
S>0
C.��ɫ��Ӧ������ȼ��ʱ������ֵ���ɫ�仯�����ڻ�ѧ�仯
D.���������绯ѧ��ʴʱ�������ĵ缫��ӦʽΪFe-3e-= Fe3+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ҫʹ��ҵ��ˮ�е��ؽ���Pb2�����ӳ��������������Ρ�̼���Ρ������������������֪Pb2����������Щ�����γɵĻ�������ܽ�����£�
| ������ | PbSO4 | PbCO3 | PbS |
| �ܽ��/g | 1.03��10��4 | 1.81��10��7 | 1.84��10��14 |
���������ݿ�֪��ѡ�õij��������Ϊ(����)������������������������������������
A������ B�������� C��̼���� D�����ϳ���������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com