
 ���ʼ��仯����ġ��й㷺Ӧ�á�
���ʼ��仯����ġ��й㷺Ӧ�á�
��1��ͬ��ʯ[��Ҫ�ɷ�]�ڸ������Ʊ����ף�P4�����Ȼ�ѧ����ʽΪ��
4Ca5(PO4)3F(s)+21SiO2(s)+30C(s)=3P4(g)+20CaSiO3(s)+30CO(g)+SiF4(g) ��![]() H
H![]()
������Ӧ�У���������������� ��
��2�������������Ϊ����������ӣ�����ṹʽ����ͼ��֮����ȥ����ˮ���Ӳ�� ��
�ṹʽΪ �����������ƣ��׳ơ����ơ����dz��õ�ˮ���������仯ѧʽΪ ��
��3���������ƣ�NaH2PO2�������ڻ�ѧ������
��ѧ��������Һ�к���Ni2+��H2PO2���������Ե������·���������Ӧ��
Ni2+ + H2PO2��+ �� Ni++ H2PO3��+
����ƽ�����ӷ���ʽ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2009?�㶫�����ʼ��仯������й㷺Ӧ�ã�
��2009?�㶫�����ʼ��仯������й㷺Ӧ�ã�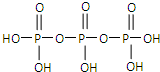
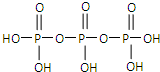
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ʼ��仯����ġ��й㷺Ӧ�ø߿���Դ��
��1��ͬ��ʯ[��Ҫ�ɷ�]�ڸ������Ʊ����ף�P4�����Ȼ�ѧ����ʽΪ��
4Ca5(PO4)3F(s)+21SiO2(s)+30C(s)=3P4(g)+20CaSiO3(s)+30CO(g)+SiF4(g) ��![]() H
H![]()
��������Ӧ�У���������������� ��
����֪��ͬ�����£�
4Ca3(PO4)2F(s)+3SiO2(s)=6Cas3(PO4)2(s)+2CaSio3(s)+SiF4(g) ����H1
2Ca3(PO4)2(s)+10C(s)=P4(g)+6CaO(s)+10CO(g)����H2
SiO2(s)+CaO(s)=CaSiO3(s) ����H3
�á�H1����H2�͡�H3��ʾ![]() H��
H��![]() H= ��
H= ��
��2�������������Ϊ����������ӣ�����ṹʽ����ͼ��֮����ȥ����ˮ���Ӳ����ṹʽΪ �����������ƣ��׳ơ����ơ����dz��õ�ˮ���������仯ѧʽΪ ��
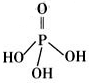
��3���������ƣ�NaH2PO2�������ڻ�ѧ������
��NaH2PO2��PԪ�صĻ��ϼ�Ϊ ��
�ڻ�ѧ��������Һ�к���Ni2+��H2PO2���������Ե������·���������Ӧ��
��a�� Ni2+ + H2PO2��+ �� Ni++ H2PO3��+
��b��6H2PO-2 +2H+ =2P+4H2PO3+3H2��![]()
���ڴ����д������ƽ��Ӧʽ��a����
�����â��з�Ӧ�������϶Ƽ�������������Ͻ𣬴Ӷ��ﵽ��ѧ������Ŀ�ģ�����һ�ֳ����Ļ�ѧ�ơ�������·���Ƚϻ�ѧ�����ơ�
�����ϵIJ�ͬ�㣺 ��ԭ���ϵIJ�ͬ�㣺 ����ѧ�Ƶ��ŵ㣺 ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��8�֣����ʼ��仯������й㷺Ӧ�á�
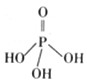
��1�������������Ϊ����������ӣ�����ṹʽ��ͼ��֮����ȥ�������Ӳ����ṹʽΪ �����������ƣ��׳ơ����ơ����dz��õ�ˮ���������仯ѧʽΪ
��2���������ƣ�NaH2PO2�������ڻ�ѧ������
��NaH2PO2��PԪ�صĻ��ϼ�Ϊ ��
�ڻ�ѧ��������Һ�к���Ni2+��H2PO2-�������Ե������·���������Ӧ��
��a��
��b��6H2PO2-+2H+ = 2P+4H2PO3-+3H2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������2010������ڶ����¿���ѧ�Ծ� ���ͣ������
��7�֣����ʼ��仯����ġ��й㷺Ӧ�á�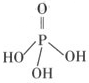
��1��ͬ��ʯ[��Ҫ�ɷ�]�ڸ������Ʊ����ף�P4�����Ȼ�ѧ����ʽΪ��
4Ca5(PO4)3F(s)+21SiO2(s)+30C(s)=3P4(g)+20CaSiO3(s)+30CO(g)+SiF4(g) �� H
H
������Ӧ�У����������������
��2�������������Ϊ����������ӣ�����ṹʽ����ͼ��֮����ȥ����ˮ���Ӳ����ṹʽΪ �����������ƣ��׳ơ����ơ����dz��õ�ˮ���������仯ѧʽΪ
��3���������ƣ�NaH2PO2�������ڻ�ѧ������
��ѧ��������Һ�к���Ni2+��H2PO2���������Ե������·���������Ӧ��
Ni2+ + H2PO2��+ �� Ni++ H2PO3��+
����ƽ�����ӷ���ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�콭��ʡ����У��һ��ѧ��������ѧ�Ծ��������棩 ���ͣ������
��8�֣����ʼ��仯������й㷺Ӧ�á�
��1�������������Ϊ����������ӣ�����ṹʽ��ͼ��֮����ȥ�������Ӳ����ṹʽΪ �����������ƣ��׳ơ����ơ����dz��õ�ˮ���������仯ѧʽΪ
��2���������ƣ�NaH2PO2�������ڻ�ѧ������
��NaH2PO2��PԪ�صĻ��ϼ�Ϊ ��
�ڻ�ѧ��������Һ�к���Ni2+��H2PO2-�������Ե������·���������Ӧ��
��a��
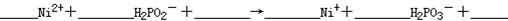
��b��6H2PO2- +2H+ = 2P+4H2PO3-+3H2��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com