
����Ŀ��ͼΪϸ����ijЩ��Ҫ�������Ԫ����ɼ����ϵ��a��b��ʾ�л�С�������ʣ�A��B�����л����������(A��ʾDNA)�� ������ش���������:

(1)ͼ��X�����Ļ�ѧԪ����____��Y��������Ҫ��ѧԪ����____�� ����b�Ľṹͨʽ��______��
(2)ͼ��A�Ǿ����������ϸ���������Ŵ���Ϣ�����ʣ��������ɵ�λa��ָ_______����____�֡�
(3)B�ж��ֹ��ܵ�ԭ�����Ϊ________________________
(4)ij����B��øˮ���õ�����Ƭ��,����һ��Ƭ�νṹ��ͼ��ʾ:
![]()
��Ƭ�ε�������____��������Ƭ��ˮ�⣬���ѵIJ�λ����Ϊ____________���ܲ���____�ְ����ᣬ��ˮ��������Է�������֮�ͱȸ����ʵ���Է�����������____��
���𰸡�N.P N ![]() ���������� 4 ����������ࡢ���������Ŀ�������������˳���Լ��������Ŀռ�ṹ���������һ���������۵���ʽ����һ�� ���� �ļ� 4 72
���������� 4 ����������ࡢ���������Ŀ�������������˳���Լ��������Ŀռ�ṹ���������һ���������۵���ʽ����һ�� ���� �ļ� 4 72
��������
�����Ĺؼ��ǶԷ���A��B��a��b��Ԫ��X��Y��ʶ��������ͼʾ��֪��B�ǵ����ʣ���Ϊͼ����B�Ĺ��ܣ���A����B������A��DNA����������C��H��O��NԪ�ع��ɣ���Щ����P��S��������λ�������ᣬԼ20�֣��ṹ�ص㣺ÿ�ְ����ᶼ���ٺ���һ��������һ���Ȼ����������Ƕ�������ͬһ��̼ԭ������
��1������ͼʾ��֪��B�ǵ����ʣ���Ϊͼ����B�Ĺ��ܣ���A����B������A��DNA����X�����Ļ�ѧԪ��ΪN��P������b�ǰ����ᣬ��Y��������Ҫ��ѧԪ����N���������ͨʽ��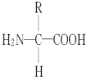 ��
��
��2��ͼ1��A��DNA���Ǿ������������Ŵ����ʣ�����ϸ���������Ŵ���Ϣ�����ʣ��������ɵ�λaָ����4��������������
��3��B�������ʣ��ж��ֹ��ܵ�ԭ������Ϊ��ɵ����ʵİ���������ࡢ���������Ŀ�������������˳���Լ��������Ŀռ�ṹ���������һ���������۵���ʽ����һ����
��4������ͼ�ο�֪��Ƭ�κ���4���ļ���CO-NH-�������Ըû�������������������Ƭ��ˮ�⣬���ѵIJ�λ����Ϊ�ļ�����ˮ������4����ˮ����������֮�ͱȸ����ʵķ�����������18��4=72������R���ŵIJ�ͬ����-CH2-C6H5��-CH3��-(CH2)4-NH2��-CH2-COOH����R���ţ���֪�û�������4�ְ�������ˮ���϶�����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʮ�Ŵ������Ҫ����Ӯ���챣��ս������ζ�ŶԴ�����Ⱦ���αȹ�ȥҪ����ߡ��������������ӽ���Ĥȼ�ϵ��ʵ���������ᡢ���硢������λһ��Ľ�ϣ�ԭ����ͼ��ʾ������˵����ȷ����

A. �õ�طŵ�ʱ���Ӵ�Pt2�缫�����ڵ�·����Pt1�缫
B. Pt1�缫���������ķ�ӦΪ��SO2��2H2O��2e����H2SO4��2H��
C. Pt2�缫���������ķ�ӦΪO2��4e����2H2O��4OH��
D. ��ͬ�����£��ŵ���������ĵ�SO2��O2�������Ϊ2��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijѧ����0.1mol/L KOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ������ɷֽ�Ϊ���¼�����
��A����ȡ20.00mL���������ע��ྻ����ƿ��������2-3�η�̪��Һ
��B���ñ���Һ��ϴ�ζ���2-3��
��C����ʢ�б���Һ�ļ�ʽ�ζ��̶ܹ��ã�����Һ��ʹ�ζ��ܼ��������Һ
��D��ȡ��KOH��Һע���ʽ�ζ�����0�̶�����2-3cm
��E������Һ����0��0�̶����£����¶���
��F������ƿ���ڵζ��ܵ����棬�ñ�KOH��Һ�ζ����յ㣬���µζ���Һ��Ŀ̶�
���������գ�
��1����ȷ������˳���ǣ��������ĸ��д��______________________��
��2��������B��������Ŀ����___________________________________��
��3��������A������֮ǰ�������ô���Һ��ϴ��ƿ����Բⶨ�����Ӱ���ǣ���ƫ��ƫС�����䣬��______________��
��4��ʵ���������ֿ���_________������������λ�����۾�ע��_______��ֱ���ζ��յ㡣�жϵ����յ��������_______________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и��ݷ�Ӧԭ����Ƶ�Ӧ��������ȷ����
A. CO32�� + H2O ![]() HCO3��+ OH�� �ȵĴ�����Һ��ϴ����
HCO3��+ OH�� �ȵĴ�����Һ��ϴ����
B. Al3+ + 3H2O ![]() Al(OH)3��������+ 3H+ ������ˮ
Al(OH)3��������+ 3H+ ������ˮ
C. SnCl2 + H2O ![]() Sn(OH)Cl�� + HCl �����Ȼ�������Һʱ������������
Sn(OH)Cl�� + HCl �����Ȼ�������Һʱ������������
D. TiCl4+ ��x+2��H2O�������� ![]() TiO2��x H2O�� + 4HCl ��TiCl4�Ʊ�TiO2
TiO2��x H2O�� + 4HCl ��TiCl4�Ʊ�TiO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и��������У���һ�����ᣬ�ڶ����ǻ����������Ǽ���ǣ� ��
A.���ᡢCuSO45H2O��������
B.���ᡢ����������
C.����������������ʯ��
D.���ᡢʳ��ˮ���ռ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ��ֲ���һ�ֲ��鶯������ϸ����ijЩ��ѧԪ�غ���(ռϸ�����ص���������)���±���ʾ�������й�������ȷ����

A. ֲ��ϸ����O�����ܸ���֬�ʺ������й�
B. �������������������Ļ�ѧԪ�ص��������ܴ�
C. �������е�N��Ҫ�������ļ���
D. �ݱ��Ʋ��ֲ��Ͷ������ڲ�������Ԫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һƿ�������Һ�����п��ܺ���NH4����K����Ba2����Al3����Fe3����I����NO3����CO32����SO42����AlO2����ȡ����Һ��������ʵ�飺
����pH��ֽ��⣬��Һ��ǿ���ԣ�
��ȡ��Һ��������������CCl4������������ˮ����CCl4����Ϻ�ɫ��
����ȡ��Һ��������μ���NaOH��Һ��
a����Һ�����Ա�Ϊ����
b����Һ��������
c��������ȫ�ܽ�
d����������Һ��������ų�����������ʹʪ��ĺ�ɫʯ����ֽ����
��ȡ�����۵õ��ļ�����Һ������Na2CO3��Һ���а�ɫ�������ɡ�
��������ʵ�����ش��������⡣
��1���ɢٿ����ų�__________�Ĵ��ڡ�
��2���ɢڿ���֤��__________�Ĵ��ڣ�ͬʱ�ų�______�Ĵ��ڣ�������_______________��
��3���ɢۿ���֤��________�Ĵ��ڣ�д��c��d���漰�Ļ�ѧ����ʽ�������ӷ�Ӧ�������ӷ���ʽ��ʾ�� c___________________________________________________ ��d___________________________________________________ ��
��4���ɢܿ����ų�__________�Ĵ��ڣ�ͬʱ֤��________�Ĵ��ڡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ֲ���纣���������к��зḻ�ĵ�Ԫ�أ���Ԫ���Ե����ӵ���ʽ���ڡ�ʵ���ҴӺ�������ȡ����������£�
����![]() �����
�����![]() ���������Һ
���������Һ![]()
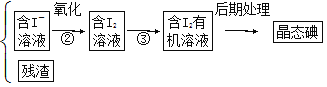
��1��д����ȡ��Ĺ������й�ʵ����������ƣ���______����________��
��2����ȡ��Ĺ����пɹ�ѡ����л��ܼ�����____��
A�����͡��ƾ� B�����Ȼ�̼������ C�����ᡢ�ƾ�
��3��Ϊ������Ϣ���������������ʵ���������ձ���������������̨����ƿ�����ܡ��ƾ��ƣ���ȱ�ٵIJ���������____________________________________��
��4���Ӻ�����л��ܼ�����ȡ��ͻ����л��ܼ�������Ҫ��������ָ����ͼ��ʾ��ʵ��װ���еĴ���֮������______����________����________����________��

��5��Ϊ���ڿ�������ʱ���¶ȣ�����ʱʹ��ˮԡ���ȣ���������________��ۼ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com